Development of Management Strategies for Herpes-like Virus Infection in Abalone
May 2009
FRDC Project No. 2006/243
ISSN 1329-7287
ISBN 978-1-74217-390-0
Author: Gavine, F., Ingram, B. and Doroudi, M.
This work is copyright. Except as permitted under the Copyright Act 1968 (Cth), no part of this publication may be reproduced by any process, electronic or otherwise, without the specific written permission of the copyright owners. Neither may information be stored electronically in any form whatsoever without such permission.
The Fisheries Research and Development Corporation plans, invests in and manages fisheries research and development throughout Australia. It is a federal statutory authority jointly funded by the Australian Government and the fishing industry.
Principal Investigator: Dr Brett Ingram
Objectives:
To develop a "Code of Practice" by improving physical, chemical and biological measures of bio-security for abalone farms to prevent the introduction and spread of the virus.
- To develop a "Code of Practice" for commercial divers to avoid the introduction and further spread of the virus in wild populations of abalone.
- To develop a "practical bio-security program" for abalone processing plants.
Non technical summary:
Outcomes achieved to date:
The implementation of the Codes of Practice developed under this project will increase bio-security in all sectors of the abalone industry (wild-capture, recreational, aquaculture and processing). As a result, the risks of further outbreaks and the spread of abalone viral ganglioneuritis will be minimised, and all industry sectors will be better prepared to respond to and control and manage the negative impacts associated with the further emergence of herpes-like virus. From the perspective of the Australian abalone aquaculture industry, the increased attention to aquatic bio-security that is required to implement their Code of Practice will result in enhanced production and sustainability because it will assist in minimising the risks of any further emergence of abalone viral ganglioneuritis. In addition, the other Codes of Practice will assist in maintaining the favourable health status of Australia's wild fisheries through protocols that will lead to improved bio-security in the wild catch, in recreational diving and fishing, and in the processing sectors.
In January 2006, a previously unknown herpes-like virus was identified as being the most likely cause of mass mortalities of abalone (Haliotis spp.) in a number of aquaculture farms in south-west and central Victoria (Hardy-Smith, 2006). The disease caused by the virus was named abalone viral ganglioneuritis. The disease was subsequently found in marine waters adjacent to one of the infected farms resulting in mortalities in wild abalone populations. The outbreak of the disease sparked concern about possible long-term consequences for the abalone industry not only in Victoria, but also in the rest of Australia. There was a clear need to develop specific management strategies which incorporated disease monitoring, detection, response and control measures in both farmed and wild stocks.
This project aims to develop Codes of Practice to standardise and improve the bio-security measures currently in place in aquaculture farms and in commercial harvesting operations and processors. For completeness, recreational divers and fishers are also included.
In developing these Codes of Practice, a robust and defensible process was undertaken to identify the key issues that need to be addressed by the wild harvest, recreational, aquaculture and processing sectors. These issues were assessed and prioritised at a Risk Assessment Workshop conducted by an expert panel assembled from all Australian states with an abalone industry. The panel included aquatic animal health specialists, representatives from industry and Government officials.
The Risk Assessment Workshop identified the most important issues for each industry sector; control measures were developed, bearing in mind that knowledge of the virus was incomplete. The controls were subsequently developed (in consultation with industry) into Codes of Practice that are comprised of a series of Standard Operating Procedures.
The adoption and implementation of the Codes of Practice by industry will increase bio-security within the Australian abalone industry and minimise the risk of further outbreaks and the spread of abalone viral ganglioneuritis.
Keywords:
Abalone, abalone viral ganglioneuritis, ganglioneuritis, bio-security, aquatic animal health.
Table of Contents
Background
- Abalone industry in Australia
- The abalone viral ganglioneuritis outbreak in Victoria
Need
Objectives
Methods
- Literature search
- Risk assessment process and development of controls
Results/Discussion
- Literature review
- Mollusc viruses
- Herpes viruses
- Abalone herpes and herpes-like viruses
- Current knowledge on the Victorian abalone viral ganglioneuritis
- Diagnostic tools to identify the virus in the future
- Potential future mechanisms for control
- Breeding for disease resistance
- Selection of spawners
- Risk Assessment Process and Workshop
- Pathways of viral transmission
- Outcomes of the Risk Assessment Workshop
- Determining control measures for the three industry sectors
- Wild abalone harvest sector
- Abalone aquaculture sector
- Abalone processing sector
Benefits and adoption
Further development
Conclusion
References
Appendix I: Summary of diseases and parasites reported from abalone
Appendix II: List of Participants
Appendix III: Risk analysis process
- Risk Ratings
Appendix IV: Risk ratings associated with spread of abalone viral ganglioneuritis
Background
In January 2006, a previously unknown herpes- like virus was identified as being the most likely cause of mass mortalities of abalone (Haliotis spp.) in a number of aquaculture farms in south- west and central Victoria (Hardy-Smith 2006). The disease caused by the virus was named abalone viral ganglioneuritis. The disease was also apparent in the marine waters adjacent to one of the infected farms and led to mortalities in wild abalone populations. The outbreak of disease sparked concern about possible long- term consequences for the abalone industry not only in Victoria, but also in the rest of Australia. Investigations were conducted into the likely source and mode of transmission of the virus. A key conclusion was that Codes of Practice should be developed to standardise and improve the bio-security measures currently in place in aquaculture farms, commercial harvesting operations and processors. This document reports the process through which the Codes of Practice were developed as part of FRDC Project No. 2006/243 "Development of management strategies for herpes-like virus infection of abalone". Although the Victorian industry will be used as a case study for the development of the Codes of Practice, the method used and key elements of the Codes of Practice are equally applicable to other states with an abalone industry.
Abalone industry in Australia
Abalone (family Haliotidae) are marine gastropods found on rocky reefs in temperate areas of Australia. Abalone form the basis of an important and valuable fishery in Australia, with a wild catch of 5,592 tonnes in 2004/05 worth around $220 million (ABARE 2006). The abalone fishery comprises around 11% (in 2004-05) of the total value of wild fisheries production in Australia (ABARE 2006). It also represents 55% (from 2001 figures) of the world wild abalone catch (Love and Langenkamp 2003). There has been a dramatic decline in global abalone production over the last three decades due to overfishing and changes in environmental and ecological dynamics (Gorfine 2002). Australia has one of the last sustainable wild abalone fisheries in the world.
There are thirteen species of abalone found in Australian waters, of which four are commercially harvested. These are:
- blacklip abalone (Haliotis rubra Leach)
- greenlip abalone (H. laevigata Donovan)
- brownlip abalone (H. conicopora Péron)
- Roe's abalone (H. roei Gray).
To ensure that the resource is not over-exploited, the abalone fishery is carefully regulated and managed in all states. Key features of the abalone fishery in each state are shown in Table 1. Tasmania has the most licence holders and the largest commercial fishery in both tonnage and value, followed by Victoria, then South Australia. Fisheries in Western Australia and NSW are relatively small compared with the other states.
In recent years, an abalone aquaculture industry has developed in several Australian states, notably Victoria, Tasmania and South Australia (Table 2). Other states have small, developing industries that have not yet reached commercially reportable quantities.
Table 1: Key features of commercial abalone fisheries production by state in 2004-05 (ABARE 2006).
| State | Species | No. Diver Licence Holders | Total catch (tonnes) | Value ($million) |
|---|---|---|---|---|
| NSW | Blacklip | 48 | 186 | 7.83 |
| Victoria | Blacklip, Greenlip | 71 | 1,491 | 60.2 |
| Tasmania | Blacklip | 123 | 2,709 | 105.4 |
| South Australia | Blacklip, Greenlip | 35 | 902 | 33.8 |
| Western Australia | Greenlip, Brownlip and Roe's | 28 | 304 | 12.65 |
| TOTAL | 270 | 5,592 | 219.88 |
Table 2: Production of farmed abalone by state (ABARE 2006).
| State | 2002-03 | 2003-04 | 2004-05 | |||
|---|---|---|---|---|---|---|
| Tonnes | Value ($M) | Tonnes | Value ($M) | Tonnes | Value ($M) | |
| Victoria | 27 | 1.2 | 102 | 3.6 | 124 | 4.5 |
| Tasmania | nd1 | 0.6 | nd | 1.6 | nd | 3.4 |
| South Australia | 59 | 3.1 | 105 | 3.1 | 177 | 5.3 |
The commercial harvesting and aquaculture sectors are supported by a vibrant abalone processing sector that prepares the abalone for sale to domestic or export markets. Processors also add value to the product through canning, or the production of other products (e.g. abalone sauce). Around 90% of abalone is sold to Asian markets. In 2004-05, a total of 4,004 tonnes of abalone were exported with a value of $263 million (ABARE 2006). This included 2,032 tonnes of fresh, chilled or frozen abalone and 1,972 tonnes of canned abalone (ABARE 2006).
As the mollusc aquaculture industry has grown in volume and importance world-wide, the incidence of disease in farming systems has also increased (Elston 1984). There are a number of factors behind this observation, including that the animals are subject to closer scrutiny when held in aquaculture farms and so diseases can be more readily identified and described. The recent outbreak of abalone viral ganglioneuritis in abalone in south-western Victoria has highlighted that Australia is not immune to such disease incursions into developing aquaculture industries.
The abalone viral ganglioneuritis outbreak in Victoria
In late 2005 and early 2006 mass mortality events occurred in abalone at several aquaculture farms in south-west and central Victoria. By May 2006, mortalities were being reported from wild abalone in the area (Hardy-Smith 2006). A previously unknown herpes-like virus was implicated as the cause. Table 3 summarises the key events in the outbreak of abalone viral ganglioneuritis in Victoria.
Mortalities were first observed in two on-shore abalone farms (Farms 1 and 2) and one sea cage farm (Farm 3). The alarm was raised in early January 2006 and samples of moribund animals were submitted for diagnostic testing. The disease was diagnosed relatively quickly and was identified using standard histology and transmission electron microscopy (TEM) techniques. The virus appears to be confined to the nervous system and causes inflammation of the nerves. For this reason the disease was initially given the name "ganglioneuritis" and was made a notifiable disease under the Livestock Disease Act 1994. In 2007, the Consultative
Committee on Exotic Animal Disease (CCEAD) decided that abalone herpes-like virus and the disease it causes should now be known as "abalone viral ganglioneuritis".
Once the virus was identified as the likely cause of the mortalities, voluntary restrictions were placed on movement of animals from the affected farms to other farms. Clinically healthy animals were sent to processing plants, with the approval of the Chief Veterinary Officer (CVO). On the farms that had mortalities from the virus, stock was culled and facilities were decontaminated. After a period of time sentinel abalone were then placed into the facilities and monitored. In November 2006, both of the land- based farms (Farm 1 and Farm 2) applied to DPI for (and were granted) permission to re-stock with abalone.
Infected abalone were first observed in the wild in the vicinity of Farm 2 (located west of Port Fairy) on 7 May 2006. By the end of May it was reported that sites east and west of the initial site were infected. On 9 June 2006, a Control Area was declared for 60-days around Port Fairy. The aim was to minimise the risk of human activity transferring the disease to unaffected abalone populations elsewhere in the state. The declared Control Area was later replaced by a Fisheries Order. An Incident Control Team was established and a structured surveillance program commenced to monitor wild abalone populations within and adjacent to the Control Area, as well as at processing plants.
Professional divers were encouraged to submit suspected cases for diagnosis. To ensure that industry and community were kept informed of the outbreak, a communication program involving regular meetings with industry and regular dissemination of updates was established. Figure 1 shows the coastal bays between Warrnambool and Port Fairy that were surveyed in the search for diseased animals. Figure 2 shows the results of that monitoring up to January 2007. Based on mortality and presence of lesions, abalone viral ganiglioneuritis was no longer detectable in areas where it had previously been present, notably in the closed area (marked in red in Figure 2). However, since tests that detect the virus if it is latent or sub-clinical are not available it is not known whether the virus is still present. In February 2007, abalone viral ganglioneuritis was also detected and confirmed in Portland Harbour west of this area.
Table 3: Herpes-virus outbreak in Victoria - summary of key events (Source: Hardy-Smith 2006, Abalone Virus - Scientific Forum, 21st September 2006, Melbourne, and other sources)
| Month/Year | Event |
|---|---|
| October / November / December 2005 | Translocation of abalone from both wild and farmed sources to and between four Victorian aquaculture farms. Some mortalities were observed in translocated stock during this period. |
| December 19th 2005 | aquaculture farms. Some mortalities were observed in translocated stock during this period. |
| December 21st 2005 | High mortalities noted in seacage system (Farm 3). |
| December 26-29 2005 | High mortalities in two broodstock tanks at Farm 2. All broodstock and abalone in two other tanks culled or harvested. No further signs of the disease. |
| Mid January 2006 | Inflammation of the nerves (abalone viral ganglioneuritis) identified by histopathological examination and herpes like virus identified within the nerve cells of abalone dying at Farm 1. Lesion and virus has never been seen before in abalone in Australia. |
| March 17th 2006 | Unusual mortalities noted at Farm 2. Mortalities rapidly escalated and spread to other tanks. |
| April 24th 2006 | Sick and dead abalone submitted from Farm 4. Abalone viral ganglioneuritis identified in four abalone. |
| May 15th 2006 | Farm 2 totally de-stocked of abalone. |
| May 18th 2006 | Abalone viral ganglioneuritis identified in some of abalone collected from the wild near Farm 2 (west of Port Fairy). |
| June 9th 2006 | Control Area declared for Western Zones 3.06 and 3.07 (surrounding Farm 2). All diving (and other proclaimed activities) prohibited in these areas, except under authority. |
| June 15th 2006 | Farm 1 totally destocked of abalone. |
| July 26th 2006 | Abalone viral ganglioneuritis confirmed in wild abalone collected from Western Zone 3.05 The Crags. |
| Early August 2006 | Mortalities reported from wild abalone 5 km west and 12 km east of initial reported site Abalone at the initial reported site showed no clinical signs of the disease. |
| September 21st 2006 | Abalone Virus - Scientific Forum (Melbourne). |
| November 2006 | Farm 1 and Farm 2 granted permission by DPI to re-stock with abalone. |
| November 2006 | Abalone viral ganglioneuritis confirmed from abalone on reef near Warrnambool, 23 km east of the initial reported site. |
| February 2007 | Disease confirmed from abalone on reef known as "Devils Kitchen", located to the west of Portland, 60 km west of the initial reported site. |
| May 2007 | Disease confirmed from beach at Murrels located on the western side of Cape Nelson. No evidence to suggest disease has progressed further east than the Warrnambool Breakwall or further west than South Bridgwater/Bully Cove. |
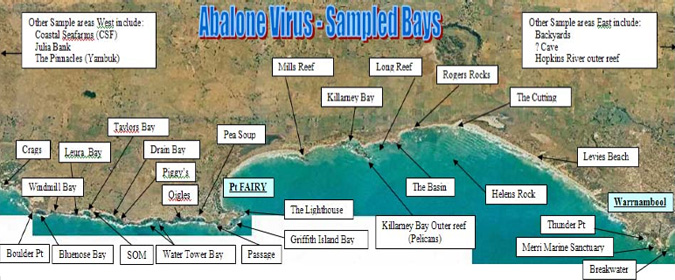
Figure 1: Bays near to Warrnambool and Port Fairy in Western Victoria that were monitored for the presence of diseased abalone (courtesy of Fisheries Victoria).

Figure 2: Results of monitoring for the virus - to January 2007 (courtesy of Fisheries Victoria).
Need
In order to protect Australia's valuable abalone industry, there is a need to develop specific management strategies which incorporate disease monitoring, detection and response and control measures in both farmed and wild stocks. It should also include the implementation of routine health management procedures and a system for exclusion of the virus. Health management strategies and Codes of Practice, for both farmed and wild abalone harvest sectors and processing plants, must be developed to improve the productivity of the farmed sector and protect the health of wild populations of abalone. To minimise the impact of the virus, the application of effective detection and exclusion (control) methods should also be incorporated into a workable bio-security plan.
Objectives
- To develop a "Code of Practice" by improving physical, chemical and biological measures of bio-security for abalone farms to prevent the introduction and spread of the virus.
- To develop a "Code of Practice" for commercial divers to avoid the introduction and further spread of the virus in wild populations of abalone.
- To develop a "practical bio-security program" for abalone processing plants.
Methods
Literature search
A search of literature was undertaken to review current information on molluscan viruses, specifically herpes viruses and herpes-like viruses, and shellfish health management, particularly related to viruses. Databases searched included:
- Aquatic Sciences and fisheries Abstracts (ASFA)
- Scopus
- Agriculture and Natural resources (ANR-Index and ANR-research)
- Environment and Natural resources (as part of ANR)
- Elixir (DPIʹs Electronic Library Exchange for Information Resources)
- Current Contents (2006 only)
- Oceanic abstracts.
General internet searches were also conducted during the review. There were 65 records related to Haliotis in ANR, of which two referred directly to aspects of health, herpes virus and herpes-like virus of molluscs with a particular emphasis on abalone.
Risk assessment process and development of controls
The risk assessment process was conducted in the following stages:
- Identification of pathways of transmission, vehicles of transmission and key risk issues. This was conducted by the project team prior to the Risk Assessment Workshop;
- Risk Assessment Workshop. Experts from all over Australia were invited to participate on the panel. The Risk Assessment was conducted according to AS/NZ 4360:2004 Standard for Risk Management.
Results/Discussion
Literature review
The recent emergence of a world-wide abalone aquaculture industry has raised awareness of various infectious diseases that are present in both wild and cultured populations of abalone. A number of international reviews have been conducted on diseases associated with abalone (Bower et al. 1994; Hine 1996; Bower 2000; 2003).
In general, relatively few abalone diseases have been identified world-wide, but some authors have suggested that this is due to a lack of examination rather than absence of disease (Handlinger et al. 2006). It is expected that as the global aquaculture industry develops more diseases will emerge, particularly if commercially important species are introduced to new geographic areas (Renault 1996).
A synopsis of currently known diseases of abalone world-wide is contained in Appendix I.
The World Organisation for Animal Health (OIE) (oie.int) recognises seven mollusc diseases that are internationally notifiable, two of which are reported from abalone (Appendix I):
- Perkinsosis caused by the protozoan Perkinsus olsoni, and
- Withering syndrome due to Candidatus Xenohaliotis californiensis, a Rickettsia-like prokaryote.
Diseases of Australian abalone have been most recently reviewed and described in a comprehensive state-by-state survey undertaken through the Abalone Aquaculture Subprogram of the Fisheries Research and Development Corporation (FRDC) (Handlinger et al. 2006). As part of this project, a national survey of diseases of commercially important abalone species was undertaken to support trade and translocation issues. The most important diseases recorded during this study were:
- Bacterial. Particularly Vibrio harveyi which has been a problem in some farms in Tasmania and South Australia (Reuter and McOrist 1999; Handlinger et al. 2005);
- Protozoans. The most important being Perkinsus olsoni. Goggin and Lester (1995) reviewed research on Perkinsus spp. in Australia and determined it is found in over 30 species of molluscs. It is recognised as a disease of wild abalone and has been brought into farms through the translocation of broodstock from infected sites.
At the time of this survey, which was conducted in 2003 and 2004, no viruses had been recorded in Australian abalone. In order to put the 2006 Victorian outbreak of abalone viral ganglioneuritis in context, a brief review of current knowledge of viruses in molluscs follows.
Mollusc viruses
Viruses are obligate intracellular parasites that depend entirely on a living host cell for essential elements of replication (Lees 2000). Viruses are the most numerous organisms in the marine environment (Fuhrman 1999) and have caused mass mortalities in a number of commercially important species around the world. Viral pathogens are often highly infectious and easily transmissible (Renault and Novoa 2004) and can cause significant losses of stocks and income in wild and farmed environments.
In general, there is limited information on mollusc viruses as research efforts have focused on viruses that are important to human health. In addition, these viruses cannot be grown in cell culture making it difficult to study them and there are other higher priority viruses on which funding has been spent e.g. avian influenza. Since many bivalves are filter feeders, they may bio-accumulate human viruses (such as hepatitis A and viruses associated with gastrointestinal diseases) especially in areas contaminated with sewage (Meyers 1984; Lees 2000). Indeed, there have been a significant number of food poisoning cases in Australia involving viruses from shellfish (Sumner and Ross 2002).
Several irido-like viruses have been reported in bivalves, notably oysters, in different countries around the world. Renault and Novoa (2004) described two distinct pathological infections in adult oysters in France:
- Gill necrosis virus (GNV) which caused mass mortalities in Portuguese oysters (Crassostrea angulata) in France; and
- Hemocyte infection virus (HIV) which was associated with mortalities of oysters in France.
In addition, oyster velar virus disease (OVVD) has caused occasional mortalities in hatchery reared Pacific oysters on the west coast of North America (Renault and Novoa 2004).
Herpes viruses
Herpes viruses (Herpesviridae) are widespread and have been recorded from a wide range of species over the world. Over 100 different herpes viruses have been described from vertebrates alone (Roizman and Baines 1991); the most notable human herpes virus is herpes simplex type 1 (HSV-1). Most agents appear to exhibit strong host specificity (Ahne 1994). The herpes viruses fall into three major groups - one associated with mammals, birds and reptiles, one with amphibians and bony fish, and a third with invertebrates (Davison 2002; Davison et al. 2005). Ahne (1994) argued that most viruses that infect aquatic poikilothermic animals, such as molluscs, do not differ in morphology and biochemical composition to those infecting terrestrial homoiothermic animals.
A summary of herpes and herpes-like viruses from aquatic invertebrates is presented in Table 4. In crustaceans, herpes-like viruses have been reported from several marine crab species. These include Callinectus sapidus, Carcinus maenas, Carcinus mediterraneus and Macropipus depurator (Johnson 1976; Bower 1995), blue crab (Paralithodes platypus) and golden king crab (Lithodes aequispina) (Sparks and Morado 1997), and Rhithropanopeus harrisii (Payen and Bonami 1979) . Ahne (1994) indicated that herpes viruses are highly pathogenic to blue crabs and blue king crabs.
In molluscs, herpes-like viruses have been reported from American oysters (Crassostrea virginica) (Farley et al. 1972), flat oysters (Ostea edulis) (Alderman 1980), and Pacific oysters (Crassostrea gigas) (Hine et al. 1992; Nicolas et al. 1992) as well as several other species.
Ostreid herpesvirus (OsHV-1)
Renault and Novoa (2004) reviewed herpes virus in Pacific oysters (Ostreid herpes virus OsHV-1 and OsHV-1 var) which exhibited a summer mortality syndrome was first described in Japan in the early 1940s. This syndrome continues to cause high mortality in Pacific oyster and other bivalves around the world (Japan, USA, Canada, China and France). While a multifactorial aetiology is suspected, the herpes virus (OsHV-1) has been mostly detected in juvenile mortality events when temperatures are high and when animals are in a relatively unstable physiological condition due to gametogenesis and spawning (ICES, 2005).
Sporadic high mortalities of larval Pacific oysters associated with OsHV-1 occur each year during summer in European hatcheries (Renault et al. 1994b; Renault et al. 2001a). The virus infects connective tissue and mantle epithelium; the velum becomes noticeably less extended and lesions appear on the velar and mantle (Nicolas et al. 1992). Larvae show reduced feeding and swimming activities and eventually mortality rates of up to 100% can rapidly occur (Hine et al. 1992; Nicolas et al. 1992). Outbreaks often occur during summer (Renault et al. 1994a; Renault et al. 1994b).
The presence of OsHV-1 has been demonstrated in asymptomatic adult Pacific oysters (Arzul et al. 2002), but adults appear to be less sensitive to infections and no abnormal mortalities have been reported (Renault and Novoa 2004). It has been suggested that the adults may act as a carrier or reservoir for the virus (Le Deuff et al. 1996; Arzul et al. 2002). Indeed, vertical transmission studies on successive generations of Pacific oysters indicate that parents pass the virus to offspring (Barbosa-Solomieu et al. 2005). However it has also been suggested that infected females may transmit some kind of protection or resistance against viral infection to their offspring (Barbosa- Solomieu et al. 2005).
Histologically, fibroblast-like cells in the connective tissue exhibit hypertrophied (enlarged) nuclei with marginated chromatin and an abnormal cytoplasmic basophilia. Nuclei in ovoid cells (haemocytes) are highly condensed (Hine et al. 1992; Nicolas et al. 1992; Renault et al. 2001a). The ultrastructure of herpesvirus in Pacific oysters has been described (Hine et al. 1992; Nicolas et al. 1992; Renault et al. 2001a).
The genome sequence for OsHV-1 has been completed and analysed (Davison et al. 2005) (GenBank No. AY509253 and NC005881). DNA sequence data demonstrated that the OsHV-1 is not closely related to herpes viruses in vertebrates (including fish), but that the herpes viruses of invertebrates form one of three major lineages of the herpes virus group. These groups may contain a large number of invertebrate herpes viruses (Davison 2002; ICES, 2003; Davison et al. 2005).
Table 4: A summary of herpes and herpes-like viruses of aquatic invertebrates.
| Name | Species | Distribution | Comments | Source |
|---|---|---|---|---|
| Molluscs | ||||
| Ostreid Herpesvirus (OsHV-1) | Crassostrea gigas | New Zealand, France, UK, USA & Mexico | Farmed hatcheries. Larvae and spat. High mortalities. May be related to poor husbandry such as elevated temperature and crowding. | Hine et al. (1992); Nicolas et al. (1992); Renault et al. (1994b); Bower (1995); Renault et al. (2001a); ICES (2004, 2005), Renault unpubl. Data, in Renault and Novoa (2004) |
| Herpes-like virus disease | Ostrea edulis | France | Cultured | Alderman (1980); Comps and Cochennect,(1993); Renault et al. (2000a) |
| Herpes-like virus disease | Crassostrea virginica | USA | Thermal effluent grow-out waters Adults. May be related to poor husbandry such as elevated temperature and crowding | Farley et al. (1972); Bower 1995 |
| (OsHV-1) | Ruditapes decussate & R. philipparum | France | Larvae | Renault et al. (2001a,b); Arzul and Renault 2002 |
| Ostrea angasi | Australia | Adults | Hine and Thorne (1997) | |
| Pectin maximus | France | Larvae | Arzul et al. (2001a) | |
| Tiostrea chilensis | New Zealand | Larvae | Hine (1997); Hine et al. (1998) | |
| Crustacea | ||||
| Bifacies virus (BFV) (=Herpes-like virus disease (HLV) | Callinectes sapidus Carcinus maenas Carcinus mediterraneus Macropipus depurator | USA & France | Death due to hemocyte dysfunction | Johnson (1976); Bower (1995) |
| "Herpes-like" hexagonal nuclear virus | Paralithodes platypus Paralithodes camtschatica Lithodes aequispina | Alaska | Extensive destruction of bladder and antennal gland | Sparks and Morado (1986); Bower (1995); Sparks and Morado (1997) |
| Rhithropanopeus harrisii | Payen and Bonami 1979 | |||
| Herpes-like virus (HLV-PA) | Panulirus argus | USA | Wild Juveniles, high mortalities, attacks haemocytes | ICES (2003; 2004) |
Molecular and immunological techniques for detection of herpes virus in Pacific oyster have been developed. These include polymerase chain reaction (PCR) and in situ hybridisation (ISH). Specific polyclonal and monoclonal antibodies have been developed and immunoassays have been validated for detection and identification of OsHV-1 (Renault et al. 2000b; Arzul et al. 2001b; Arzul et al. 2002; Lipart and Renault 2002; Renault 2003; Renault et al. 2004; Batista et al. 2005). Development of immunochemistry and ELISA tests for OsHV-1 will also be possible when cloned sequences, which enable the synthesis of recombinant virus proteins, become available (Renault and Novoa 2004). These tests may have some relevance to diagnosing abalone viral ganglioneuritis.
Unlike vertebrate herpes viruses, which are generally confined to a single host species, OsHV-1 can infect several bivalve species and is probably responsible for all the infections observed in European bivalves species (Le Deuff et al. 1994; Arzul et al. 2001c; Arzul et al. 2001b; Renault et al. 2001a; ICES, 2006). Indeed, Arzul et al. (2001c) suggested that intensive culture of bivalves in hatcheries, where there is large numbers and a variety of species in close proximity, may promote interspecies transmission.
In summary, herpes viruses have caused massive losses in oyster hatcheries in Europe, USA and New Zealand and the relevant literature is summarised in Table 4.
Herpes viruses are also reported from fish, notably Channel catfish herpes virus (CCV), Anguilla herpes virus, carp herpes virus (Ahne 1994) and pilchard herpes virus, which on a number of occasions has caused high mortalities of pilchards (Sardinops sagax neopilchardus) over their entire geographical range on the Australian coastline (Crockford et al. 2006).
Abalone herpes and herpes-like viruses
The Australian abalone herpes-like virus (which caused the disease known as abalone viral ganglioneuritis) is not limited to one species. It has been confirmed in two species (H. rubra and H. laevigata) and their hybrids. It is not known if the virus is already capable of (or will mutate to a form that is capable of) infecting other molluscan species in the region, in particular other commercially important species such as Pacific oysters (C. gigas) and mussels (Mytilus edulis). A summary of the viruses reported from abalone is presented in Table 5. Prior to the outbreak of abalone viral ganglioneuritis, herpes viruses had been recorded in abalone in Taiwan.
Herpes viruses of abalone in Taiwan
The abalone Haliotis diversicolor supertexta is the main gastropod farmed in Taiwan. Early this century, the annual production value of the industry was estimated to be in excess of US$200 million (Chang et al. 2005). In January 2003, high mortalities of abalone occurred among both sea and land farms in north-eastern Taiwan (Chang et al. 2005), though die-offs apparently first occurred two years prior to this event (Chiu Yu- Tzu 2003). These mortalities resulted in losses of US$11.5 million to the domestic abalone industry.
As a direct result of the die-off market, value dropped from NT$600 to NT$400 per kilogram (Chiu Yu-Tzu 2003). The impact of this outbreak is evident in production figures for famed abalone in Taiwan leading up to 2003 (Figure 3).
Key features of the Taiwanese virus included (after Chang et al. 2005 and Pen Heng Chang, pers comm. 2006):
- Highest prevalence occurred during winter months (water temperatures were 16-19°C). High mortalities also occurred in 2004 at some previous virus-free farms where water temperature was 18°C or less in February (Pen Heng Chang, pers comm. 2006)
- Both juveniles and adults were affected, mortalities up to 80% and death within 3 days of onset of clinical signs. Wild abalone beside the ponds also died
- Japanese black abalone (H. discus) and Trochus spp. held in ponds with infected H. d. supertexta remained normal
- Gross appearance: mantle recession and muscle stiffness
- Histopathology showed that nerve tissue was the primary target. Lesions involving nerve tissue and surrounding muscles occurred in the foot. Lesions were also evident in the oesophagus and intestine
- Electron microscopy showed hexagonal viral particles (90-100 nm) within the degenerated cells of the cerebral ganglion
- Laboratory infection trials indicated horizontal transmission and a high degree of virulence with 100% mortality.
Table 5 :Viral diseases reported from Abalone (source: Bower et al. 1994; Hine 1996; Bower 2000; 2003; Handlinger et al. 2006; Jones 2006 and pac.dfo-mpo.gc.ca/sci/shelldis/)
| Disease/parasite | Species | Location | Source | Size | Comments |
|---|---|---|---|---|---|
| Herpes-like virus disease | H. diversicolor supertexta | Taiwan | Cultured & wild | Juveniles & adults | Serious disease causing mass mortality (Chang et al. 2005) |
| Spherical Virus | H. diversicolor supertexta H. discus hannai | China | Cultured | All sizes | Serious disease causing high mortalities. Associated with "crack-shell disease". Causes necrosis and disorders of connective tissues of all organs (see Wang and Li 1997; Li et al. 1998; Huang et al. 1999; Song et al. 2000; Wang et al. 2000; Zhang et al. 2001; Shi Zhengli and Handlinger 2004; Wang et al. 2004) |
| Infectious amyotrophia (Virus-like particles) | H. discus discus H. discus hannai Nordotis madaka | Japan | Cultured | Juveniles | Serious disease causing muscle atrophy in the mantle and foot resulting from tumours of the pedal ganglia and nerve trunks which impedes feeding and adhesion to the substrate. Impaired shell growth and mortality follows. Agent is unknown, but retrovirus-like particles were observed. Epizootic mass mortalities attributable to this disease have been observed. Usually occurs at 18 to 20 °C (see Otsu and Sasaki 1997; Momoyama et al. 1999; Nakatsugawa et al. 1999; Nakatsugawa et al. 2000) |
| Intestinal viral inclusions I - GIT | H. rubra | Australia (Tas & WA) | Cultured & wild | Putative viral infection (Handlinger et al. 2006) | |
| Intestinal viral inclusions II - Granulocytes | Australia (Tas) | Wild | Putative viral infection (Handlinger et al. 2006) |
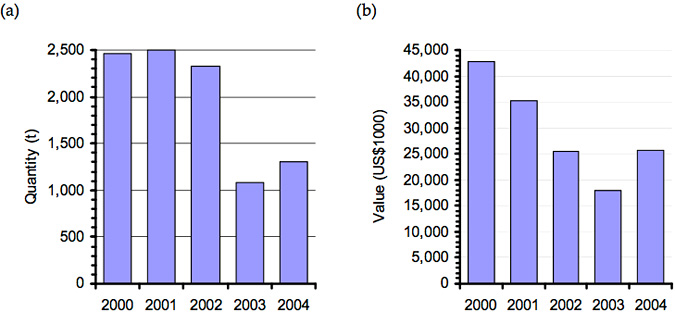
Figure 3: Change in quantity (a) and value (b) of farmed abalone Haliotis spp. (excluding H. rubra, H. midae and H. tuberculata) in Tawain between 2000 and 2004 (Data source: fao.org/figis)
This was apparently the first reported case of herpes virus in abalone. Chang et al. 2005 suggested the disease was an exotic pathogen, perhaps introduced with imported juvenile abalone. There is no documented management strategy for the Tawain abalone virus. Current control measures for the disease focus on prevention (Pen Heng Chang, pers comm. 2006). These include reducing stress factors in farmed stock, such as lower stocking densities, and prompt removal of mortalities from ponds. In the epizootic area, soda lime is applied to the culture ponds following each harvest. Quarantine is important as workers, equipment and tools may spread the virus to different farms.
Other abalone viruses
In terms of viruses that cause mass mortalities in abalone, a number of serious cases have been reported from China's farmed abalone (Table 5). "Crack-shell" disease of H. discus hannai was reported as being caused by a spherical virus of 150-220nm in size (Wang et al. 2000). It existed in the cytoplasm of cells rather than the nucleus. The virus mainly invaded foot muscle, mantle, gonads, liver and gills. The symptoms of sick abalone included: edge of front shells turned inside out, breathing holes melted into each other, decreased activity, and the soft body became slim and gradually died.
Shi Zhengli and Handlinger (2004) described an abalone viral mortality that encompassed both crack-shell disease of Haliotis hannai and viral disease of H. diversicolor. The authors noted that these two syndromes could be distinct diseases, but further information was required to make that determination. The authors described four types of spherical virion, all less than 150 nm in size. The type 1 virus was present in the cytoplasm of haemocytes and connective tissues whereas types 2-4 were usually present in the cytoplasm of epithelial and connective cells of liver and intestines (Shi Zhengli and Handlinger 2004). Infection with type 1 virus resulted in low activity, lost appetite, unresponsive to light, thin shell with the edge turned down and decreased growth rate. Virus type 2-4 is characterised by the secretion of mucus, loss of appetite and low activity, contracted feet and mantle, black and hardened feet.
Amyotrophia (Epizootic fatal wasting disease) was first reported in the early 1980s from a number of culture facilities growing Japanese black abalone (H. discus discus) in western Japan. Mass mortalities occurred in juveniles at temperatures of between 18-20°C (Otsu and Sasaki 1997). H. discus hannai, has since also been found to be vulnerable to amyotrophia (Nakatsugawa et al. 1999). Infected abalone develop tumours and muscle atrophy in the mantle and foot, especially near the nerve trunk of the pedal ganglia and their transverse commissures. Feeding and adhesion to the substrate is impeded and shell growth is impaired. Laboratory experiments have shown that the disease can be horizontally transmitted through the water column (Nakatsugawa et al. 2000). The etiological agent of amyotrophia is unknown. Virus-like particles have been observed in the cytoplasm of cells near the nerve trunk (Otsu and Sasaki 1997) and haemocytes (Nakatsugawa et al. 1999) of diseased Haliotis discus discus. Virus-like particles from haemocytes were retrovirus-like in size and morphology (Nakatsugawa et al. 1999).
Currently there are no known methods of prevention or control. Management focuses on avoiding the risk of introducing this pathogen to other culture facilities or natural stocks. Only animals certified to be free of infection should be considered for transplantation from areas where the disease occurs. In addition, imported animals must be held in quarantine and assayed for cryptic or subclinical infections prior to release into the new environment (Bower 2001).
Current knowledge on the Victorian abalone viral ganglioneuritis
Abalone viral ganglioneuritis causes damage to the nerve tissues of abalone (the ganglion) and results in death in most cases. It is known to affect greenlip, blacklip and hybrid abalone and it has been observed that young stock are more seriously affected than adult stock. The incubation period of the virus is between 2-14 days with significant mortalities occurring after 10-14 days. The virus is noticed most often where there are dense populations of abalone, but the animals may be pre-disposed to becoming infected if other stress factors are present, including:
- High stocking densities
- High water temperatures >17.5-18°C which are at the upper limit of optimal for abalone culture
- Spawning activity
- Concurrent disease (e.g. Vibrio sp.).
Clinical signs
Infected abalone are lethargic and may have enlarged mouth-parts and a curled foot. The presence of enlarged mouthparts in isolation is not sufficient to diagnose abalone viral ganglioneuritis and is not shown by all infected abalone. In some cases, the radula (a band of sharp hooked teeth) may protrude from the mouth. When clinically affected by the virus, the abalone are weak and are easily removed or fall off the substrate. In tanks on aquaculture farms, where the abalone can be more closely monitored than animals in the wild, mortalities of 5% to 90% have occurred.
Initial identification
Abalone viral ganglioneuritis was initially observed in infected abalone using light microscopy (Hardy-Smith 2006). Samples of infected tissues were subsequently submitted to the Australian Animal Health Laboratory (AAHL) where Electron Microscopy was used to examine the specimens. Viral particles were visualised in pleuropedal ganglion and, morphologically, these particles resembled viruses from the family Herpesviridae (Hardy- Smith 2006).
Transmission of the virus
Trials at AAHL have shown that the virus is transmitted horizontally through the water column and does not require direct contact for transmission (Hardy-Smith 2006). The virus is highly pathogenic, killing all healthy experimental animals within six days of exposure to the virus (by injection and co- habitation). The virus remains virulent and pathologic after being frozen at -80°C. An experiment in which healthy animals were placed in water spiked with various dilutions of virus inoculum indicated that the virus remained infectious even after a 1 in 100 dilution. There is a marked decrease in virulance with dilution.
Knowledge gaps
In September 2006, a series of workshops were held with international and Australian experts on aquatic animal diseases (Table 6) and other stakeholders in the abalone industry. These workshops were organised by the Victorian Department of Primary Industries and the commercial abalone harvesting sector in a process separate to this project. As an outcome of the workshops, the following key knowledge gaps on abalone viral ganglioneuritis were identified:
- Origin: It is not known whether the virus is exotic or endemic to Australian waters, but the panel strongly suspected that it is endemic as no probable linkages with an exotic source had been identified.
- Range: If the virus is endemic, it is not known if the outbreak is localised (as a result of translocating infected animals) or if the virus is widespread, but reached a critical dose when concentrated on the farm. Diagnostic techniques (e.g. PCR) must be developed and validated before the distribution of the virus in Australian waters can be determined.
- Mode of action: Very little is known about how this virus infects abalone, what the infectious dose is, how long it survives outside of the host (and in the water column) and if healthy abalone can carry the virus without showing symptoms.
- Characterisation of the virus: The virus has not been fully characterised. Key information on the types of cell infected is limited. It is suspected that mucous cells can carry the virus, but it is not known how important mucus and physical strands of decaying tissue are in disease transmission. Determining the relationship of the Australian abalone viral ganglioneuritis to other herpes viruses of abalone is also important.
- Other species affected: It is not known if the virus is specific to abalone. It is also not known if the virus infects other species or if other species acts as carriers of the virus.
- Deactivation of the virus: There is no specific information on what de-activates the virus. It is not currently possible to conduct research on which detergents or disinfectants are effective against this virus. The thermal tolerance range of the virus is unknown although it is known to survive freezing to - 80°C (M. Lancaster, pers. comm.).
- Mechanism of the disease. Once an animal is infected the disease can spread rapidly, particularly if there are dense populations of abalone. It is not known if the rapid time frame is determined by rapid multiplication or an expression of some other response.
Table 6: Some of the International and Australian Experts involved in the gap analysis.
| Country of Origin | ||
|---|---|---|
| International experts | Dr Anna Mouton Dr Carolyn Frideman Dr Mike Hine Dr Tristan Renault | South Africa USA New Zealand France |
| Australian experts | Dr Judith Handlinger Dr Mehdi Doroudi Dr Sally Ridge Dr Paul-Hardy-Smith Dr Jeremy Prince | Tasmania PIRVic, DPI Biosecurity Victoria Panaquatic Health Solutions Pty. Ltd Biospherics P/L |
Diagnostic tools to identify the virus in the future
The diagnostic tools currently available to identify the virus are histopathology and electron microscopy (EM). EM is labour intensive, time consuming and can only focus on a very small piece of abalone tissue at a time. However, using EM, pathologists can visualise virus particles in tissues. Histopathology is more suited to screening larger numbers of abalone. This is a field of pathology which specialises in the study of the structure of diseased tissue. If the animal is clinically infected with the virus then signs of the virus can be detected within the nerve cells (e.g. inflammatory changes).
For both tools, the tissue will be processed using special chemicals and fixatives. Using histopathology, the tissues will be examined under a light microscope by an experienced pathologist. However, if the animal is infected but ganglioneuritis has not developed or the virus is latent, then it cannot be detected using histopathology. It is highly unlikely that it will be detectable using EM. The development of more sensitive and specific tests will be required to learn more about the nature of abalone viral ganglioneuritis, and diagnosis of it in animals not exhibiting clinical signs of disease.
Options for detection of the virus in abalone need to be rapid, cost effective and ideally non- destructive. Two options are available:
Molecular tests
A range of molecular diagnostic technique have been developed for fish and shellfish including polymerase chain reaction (PCR) amplification of nucleic acids, restriction enzyme analysis, probe hybridisation and nucleotide sequencing (Cunningham, 2002).
PCR is a rapid diagnostic technique that will provide a result within days. PCR amplifies DNA, is very specific and very sensitive and has been used to investigate herpes virus infections in Pacific oyster larvae (Renault and Arzul, 2001). The test will indicate the presence of the viruses' DNA. However, because the test cannot determine whether the virus is viable or not, there is a risk of false positives. PCR needs to be done in conjunction with other testing methods such as in-situ hybridisation and immunohistochemistry.
Culture agent
Some viruses are maintained in the laboratory on cells lines of the host. Marine invertebrate cells are maintained in primary cultures with limited or no growth, surviving for periods ranging from a few hours to several months. No immortal marine invertebrate cell lines have been established. There are no cell lines for abalone. Attempts to grow OsHV-1 in the laboratory on fish cell lines and a gastropod cell line have failed. There are gastropod cell lines, but it is uncertain if herpes virus can be cultured on these lines. Abalone viral ganglioneuritis virus so far cannot be grown on available fish cell lines at AAHL (M. Crane, pers com.).
Potential future mechanisms for control
Breeding for disease resistance
Through various selective breeding programs a number of mollusc species have improved resistance to several important diseases. Some examples of resistance include: Sydney rock oysters to QX disease and winter mortality (Nell and Perkins 2006), eastern oysters (Crassostrea virginica) to MSX (Allen 1998), and European flat oysters (Ostrea edulis) to Bonamia (Naciri-Graven et al. 1998).
Selection of spawners
Another potential control measure involves collecting and using virus-free broodstock based on the detection of the pathogen by PCR. This has served as an effective control measure against vertical transmission of viral nervous necrosis (VNN) in Japanese parrotfish and striped jack and panaeid acute viremia (PAV) (Muroga 2001).
Risk Assessment Process and Workshop
A Risk Assessment Workshop was convened to identify and prioritise the risks associated with the transfer of abalone viral ganglioneuritis between the abalone aquaculture industry, commercial fishing and processing sectors. The Workshop was held on 3 October 2006 at Attwood, Victoria. A panel of experts and industry stakeholders from all over Australia was invited to participate (Appendix II). The risk assessment process followed is outlined in Appendix III and is consistent with the AS/NZ 4360:2004 Standard for Risk Management.
Issues were assessed using a consequence - likelihood score. The issues were assigned a risk category according to the score given by the panel:
- High risk - scored between 16 and 25.
- Significant risk - scored between 11 and 15.
- Moderate risk - scored between 5 and 10.
Pathways of viral transmission
Prior to the workshop, the project team identified potential pathways through which the herpes- like virus could be transmitted between the three key sectors under investigation (Figure 4).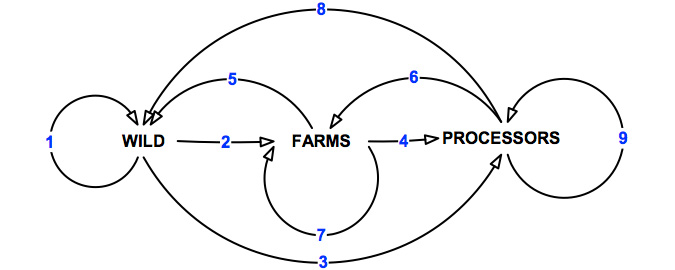
Figure 4: Potential pathways of transmission of abalone viral ganglioneuritis.
For the purposes of this exercise, "wild" is primarily activities associated with commercial diving activities. However, this could also incorporate other commercial fishing and recreational activities (e.g. recreational diving, fishing) as well as illegal take of abalone2. There may also be some overlap with sea-cage farming, ranching and stock enhancement programs. There are nine primary pathways of transmission of abalone viral ganglioneuritis between the three industries sectors (Table 7).
Table 7: Potential pathways of transmission for abalone viral ganglioneuritis.
| Pathway | Description |
|---|---|
| 1 | Movement within the wild sector. |
| 2 | Movement from the wild to abalone farms. |
| 3 | Movement from wild to processors. |
| 4 | Movement from abalone farms to processors. |
| 5 | Movement from abalone farms to wild sector. |
| 6 | Movement from processors to abalone farms. |
| 7 | Movement within and between abalone farms. |
| 8 | Movement from processors to wild sector. |
| 9 | Movement within and between processors. |
Once the pathways of transmission had been identified, it became clear that within each pathway there were common mechanisms through which the virus could be spread. These included:
- Movements of live infected abalone.These movements may be intentional (e.g. an abalone farm procuring new broodstock from the wild, a commercial diver transferring harvested abalone to a processor) or unintentional (e.g. escape from an abalone farm). It should be noted that abalone may carry the virus but show no symptoms. It should also be noted that a live abalone infected with abalone viral ganglioneuritis may be constantly or intermittently secreting the virus particles (as compared to dead abalone, personnel or equipment where the defined quantity of virus will diminish over time).
- Movements of dead infected abalone, abalone by- products and wastes. This includes mortalities from abalone farms, shells and processing wastes (viscera etc).
- Personnel and equipment. Transfer of the virus on contaminated personnel and equipment. This includes transport bins and tanks, boats, wetsuits, buckets, nets and boots. This category also includes abalone diets. As noted above the virus cannot multiply on personnel or equipment, hence the quantity of viable virus will diminish over time on such vectors.
- Water. Transfer of the virus in contaminated water. This may include water pumped from an infected reef to an abalone farm, water discharged from an abalone farm, boat and ship bilge and ballast water, water used in the holding and transport of live abalone and water used in processing plants. Laboratory experiments have shown that transmission of the abalone viral ganglioneuritis can occur through the water column (horizontal transmission). Viability of the virus in the environment outside the host is unknown.
- Other animal species. A number of other species may act as carriers or vectors of the abalone viral ganglioneuritis. These include other gastropods, crayfish, vermin (e.g. rats), predators (e.g. birds, fish) and scavengers.
The relationships between the mechanisms of virus movement and the three industry sectors are complex and interlinked. In undertaking the risk analysis, each pathway represented a risk category while the specific issues within each pathway are represented by mechanisms of virus movement. Specific risks also take into account geographic scales of movement within and between the industry sectors. While the current risk assessment focused on the above nine pathways, it is likely there are other pathways through which the viable virus particles may be spread (Figure 5). It is also possible that the current pathways and associated risks will change as knowledge and understanding of the virus (e.g. infective dose, environmental stability) increases.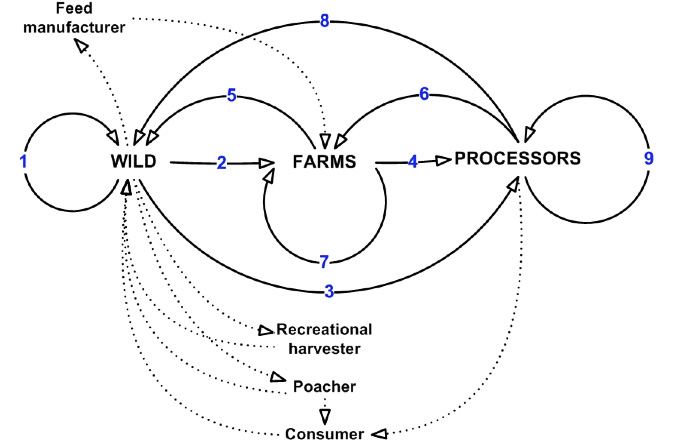
Figure 5: Additional pathways of transmission of abalone viral ganglioneuritis in abalone and associated industries.
Outcomes of the Risk Assessment Workshop
Appendix IV shows that a total of 84 specific risks were identified for the nine pathways. Error! Reference source not found. Some issues raised at the workshop that did not fall within any of the nine pathways were summarised in a category named "Other". Figure 6 illustrates that the highest number of specific risks were in Pathway 7 (20%) (movement within abalone farms) and the least in Pathway 8 (5%) (movement from processors to the wild).
The outcome of the risk assessment process was as follows:
- 24 risks rated as High
- 20 rated as Significant
- 28 rated as Moderate.
More than half (52%) of the specific risks were associated with movement of infected live abalone (Figure 7), which occurred in all pathways, but especially within Pathway 7. Approximately 50% of the specific risks were associated with local-regional (i.e. within and between management zones) movements. A complete list of specific risks for each pathway, including likelihood, consesquence and ratings scores and associated comments, is presented in Appendix IV.
Herpes-like virus risk ratings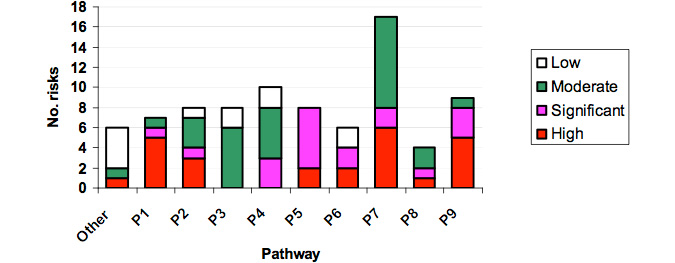
Figure 6: Number of risks recorded for each Pathway of Transmission of abalone viral ganglioneuritis.
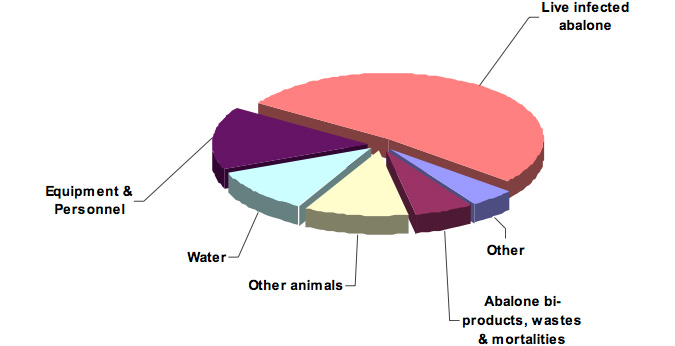
Figure 7: Proportion of risks for each of the primary vehicles of transport of abalone viral ganglioneuritis.
Determining control measures forthe three industry sectors
Wild abalone harvest sector
The "wild harvest" abalone sector as defined in this document is primarily associated with commercial harvesting activities. However, this could also incorporate other commercial fishing and recreational activities (e.g. recreational diving, abalone poaching and fishing). There may also be some overlap with sea-cage farming, ranching and stock enhancement programs. The pathways of transmission over which the wild sector has control are shown in Figure 8 and detailed in Table 8.
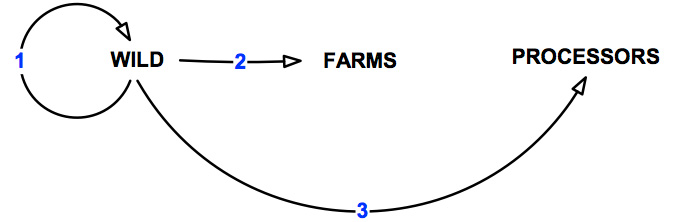
Figure 8: Pathways of transmission associated with the wild abalone harvest sector.
Table 8: Pathways of transmission associated with the wild abalone harvest sector.
| Pathway | Description | Number of risks assessed |
|---|---|---|
| 1 | Movement within the wild | 7 |
| 2 | Movement from the wild to abalone farms | 9 |
| 3 | Movement from wild to processors | 7 |
| All | Total | 23 |
High risk issues related to the wild abalone harvest sector (all pathways)
Table 9 shows that six "High" risk issues were identified across all of the relevant pathways at the workshop. Possible controls for these risks are also shown in Table 9.
Significant risk issues related to the wild abalone harvest sector (all pathways)
Table 10 shows that three "Significant" risk issues were identified across all of the relevant pathways at the workshop. Possible controls for these risks are also shown in Table 10.
Moderate risk issues related to the wild abalone harvest sector (all pathways)
Table 11 shows that nine "Moderate" risk issues were identified across all of the relevant pathways at the workshop. Possible controls for these risks are also shown in Table 11.
Table 9: "High" risk issues associated with the wild abalone harvest sector.
| Pathway | Issue | Controls |
|---|---|---|
| 1. Wild to wild | Movement of contaminated equipment and personnel by commercial divers from infected reef/zone to un-infected reefs/zones. Movement of contaminated equipment and personnel by other human activities including:
| Restrict entry to "infected areas" through declaration of an area of control via a Fisheries Notice or other such instrument. Where the status of a reef is "infected", disinfect equipment before moving to another reef. |
Movement of virus contaminated water between reefs/zones (transfer of virus particles in current). Either passive movement in water currents or deliberate discharge of tank/bilge/ballast water. Caught abalone are cooled on boats and water runs off to the sea. | No practical controls for passive movement of water between reefs/zones. Where the disease status of the reef is unknown, abalone must be cooled on board ship where they are harvested. Once the vessel is moving, discharge of water must stop. Disinfection of bilge water. | |
Sea-ranching. Deliberate movement of live infected animals between reefs for sea- ranching purposes. | Sea-ranching controlled by the relevant Fisheries Agencies. Applications dealt with on a case-by case in each state. Affected reefs or animals should be excluded. | |
| Movement of the virus from infected reef/zone to an un-infected reefs/zones by other animals (carriers, vectors, predators and scavengers). | No practical controls. | |
| 2. Wild to farm | Movement of equipment and farm personnel introduces disease onto aquaculture farm e.g. recreational diving, sharing/storage of diving equipment, divers servicing farm water intakes, broodstock collection and visitors to farms. | Aquaculture farmers must restrict movements of staff and equipment between the wild and farms. Follow bio-security protocol for aquaculture regarding equipment and people moving onto farms. |
Table 10: Significant risks associated with the wild abalone harvest sector.
| Pathway | Issue | Controls |
|---|---|---|
| 1. Wild to wild | Natural movement of live infected animals (all life stages) between reefs/zones. Will depend on distance between reefs. Assumes infected abalone have the capability of crawling between reef system and the disease is vertically transmitted through settling spat. | No practical controls. |
| 2. Wild to farm | Movement of live infected animals from an infected reef to a farm in an adjoining zone in Victoria. Mainly adult animals. | Abalone broodstock collection controlled by the relevant Fisheries Agencies through Broodstock Collection Permits. Abalone broodstock collection controlled by the relevant Fisheries Agencies through Broodstock Collection Permits. Prior to translocation, the permit holder must observe the health status of abalone on the reef. Divers should follow protocol for identifying animals with herpes- like infection and report any unusual signs of mortalities or disease to the relevant authority. Abalone may not be collected from reefs with an "infected status" as defined under the state Disease Control legislation. Aquaculture licence holders must comply with quarantine procedures in the bio-security protocol. |
Table 11: "Moderate" risk issues associated with the wild abalone harvest sector.
| Pathway | Issue | Controls |
|---|---|---|
| 1. Wild to wild | Deliberate movement of live infected animals between reefs (recreational activities, restocking/re-seeding programs & bioterrorism). | Sea-ranching controlled by the relevant Fisheries Agencies. Applications dealt with case-by case in each state. Affected reefs or animals should be excluded. No practical controls (except communication). |
| 2. Wild to farm | Movement of live infected animals from an infected reef to an adjacent farm - local movements Movement of live infected animals from wild to interstate farm Movement of live (wild) infected animals from to a farm in the same state. | Divers should follow protocol for identifying animals with herpes-like infection. Infected animals should not be removed from reefs except as samples for diagnostic testing. No infected animals are to be moved to farms. Following identification of infected status all boats, equipment and personnel must be decontaminated. |
Introduction of other animal species that may harbour the virus from an infected reef/area onto a farm. | All boats, equipment and personnel must be decontaminated. Farm bio-security protocol, particularly quarantine protocol must be followed. | |
| 3. Wild to processor | Movement of live infected animals (abalone and other species that may harbour the virus) from wild to processor - local movements. Movement of live infected animals (abalone and other species that may harbour the virus) from wild to processor - regional movements. Movement of live infected animals (abalone and other species that may harbour the virus) from wild to processor - interstate movements. Movement of abalone transport containers introduces disease from wild into processor. | Divers should follow protocol for identifying animals with herpes-like infection. Infected animals should not be removed from reefs except for samples for diagnostic testing. No infected animals should be moved to processors. Following identification of infected status all boats, equipment and personnel must be decontaminated. All transport containers and vehicles must be decontaminated. |
Abalone aquaculture sector
The pathways of transmission over which the abalone aquaculture sector has control are shown in Figure 9 and detailed in Table 12.
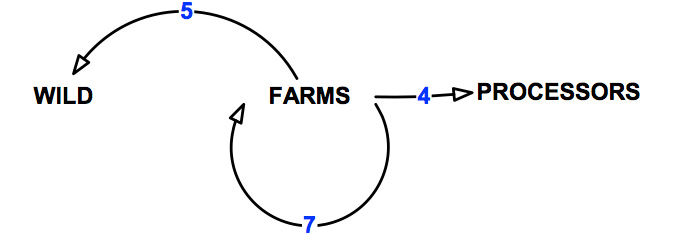
Figure 9: Pathways of transmission associated with the abalone aquaculture sector.
Table 12: Pathways of transmission associated with the abalone aquaculture sector.
| Pathway | Description | Number of risks assessed |
|---|---|---|
| 4 | Movement from abalone farms to processors. | 10 |
| 5 | Movement from abalone farms to the wild sector. | 8 |
| 6 | Movements within and between abalone farms. | 17 |
| All | Total | 37 |
High risk issues related to the abalone aquaculture sector (all pathways)
Table 13 shows that eight "High" risk issues were identified across all of the relevant pathways at the workshop. No high risk issues were identified for Pathway 4 (Movements from abalone farms to processors). Possible controls for these risks are also shown in Table 13.
Significant risk issues related to the abalone aquaculture sector
Table 14 shows that 10 "Significant" risk issues were identified across all of the relevant pathways at the workshop. Possible controls for these risks are also shown in Table 14.
Moderate risk issues related to the abalone aquaculture sector (all pathways)
Table 15 shows that 11 "Moderate" risk issues were identified across all of the relevant pathways at the workshop. Possible controls for these risks are also shown in Table 15.
Table 13: "High" risk issues associated with the abalone aquaculture sector.
| Pathway | Issue | Controls |
|---|---|---|
| Farms to wild | Intentional movement of live infected farmed animals from farm to wild in another zone. Intentional interstate movement of live infected farmed animals to the wild. | For example, seacage farming or sea-ranching/stock enhancement. Implies farmer knows that the animals are infected prior to translocation. Controls include:
|
| Farm to farm | Movement of live infected farmed abalone from farm to farm - adjoining zones within state. Movement of live infected farmed abalone from seacage to seacage - adjoining zones within state. Movement of live infected farmed abalone from farm to farm - interstate. Movement of live infected farmed abalone from seacage to seacage - interstate. Movement of live infected farmed abalone from farm to farm - international Movement of live infected farmed abalone from seacage to seacage - internationa |
Table 14: "Significant" risks associated with the abalone aquaculture sector.
| Pathway | Issue | Controls |
|---|---|---|
| Farm to processor | Movement of live infected farmed animals from farm to live/holding processor - regional movement. Movement of live infected farmed animals from Victorian farm to interstate holding processor. | Abalone infected with herpes virus should not be moved under any circumstances. Farm should implement a health surveillance program and batch certification as part of the application for translocation approval. If herpes virus infection is confirmed, the relevant state agency must be notified according to the farm's health management plan. |
| Farm to wild | Unintentional movement of live infected farmed animals from farm to wild (escape of farmed stock). | Farms should comply with bio-security protocol - prevention of escapes. |
Intentional movement of live infected farmed animals from farm to wild in same zone. | Abalone infected with herpes virus should not be moved under any circumstances. Farm should implement a health surveillance program and batch certification as part of the application for translocation approval. | |
Movement of contaminated equipment and farm personnel introduces disease into wild. Discharge of virus infected effluent water may transfer disease to adjacent reefs. | Farms should comply with bio-security protocol - preventing spread of infection. | |
Transfer of virus to uninfected wild areas through inappropriate disposal of viscera, shells and mortalities from farms. | Farms should dispose of viscera, shells and mortalities appropriately. | |
Transfer of virus from farm to wild by other animals that may harbour the virus (e.g. predators/scavengers). | Farms should comply with bio-security protocol. | |
| 7. Farm to farm | Movement of live infected farmed abalone from sea-cage to seacage - local movements. Movement of live infected farmed abalone from farm to farm - local movements. | Abalone infected with herpes virus should not be moved under any circumstances. Farm should implement a health surveillance program and batch certification as part of the application for translocation approval. Quarantine of new stock. |
Table 15: "Moderate" risk issues associated with the abalone aquaculture sector.
| Pathway | Issue | Controls |
|---|---|---|
| Farm to processor | Movement of live infected farmed animals from farm to live/holding processor - local movement. Movement of contaminated equipment and personnel from farm introduces disease into processor. | Staff trained to identify clinical signs of virus. Infected animals should not be moved to processor. Follow processor protocol. |
| Farm to farm | Movement of live infected farmed abalone between areas within the farm introduces virus to other parts of farm. Movement of contaminated equipment and personnel introduces virus to other parts of farm. Movement of contaminated equipment and personnel transfers virus seacage to seacage. Movement of contaminated equipment and personnel transfers virus between farms. Movement of water may carry virus particles within farms. Movement of water may carry virus particles between farms. Movement of other species that may harbour the virus within farm. Movement of other species that may harbour the virus from seacage to seacage. Movement of other species that may harbour the virus from seacage to seacage. | Farms should comply with bio-security protocol. |
Abalone processing sector
The pathways of transmission over which the abalone processing sector has control are shown in Figure 10 and detailed in Table 16.
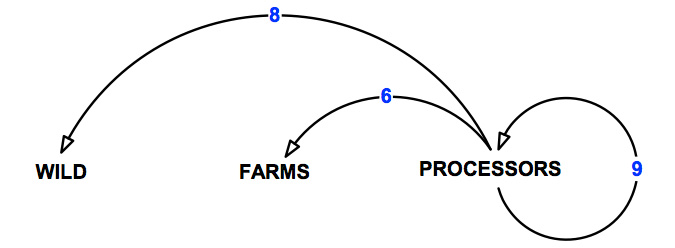
Figure 10: Pathways of transmission associated with the abalone processing sector.
Table 16: Pathways of transmission associated with the abalone processing sector.
| Pathway | Description | Number of risks assessed |
|---|---|---|
| 6 | Movement from processors to abalone farms. | 6 |
| 8 | Movements from processors to the wild. | 4 |
| 9 | Movements within and between processors. | 9 |
| All | Total | 19 |
High risk issues related to the abalone processing sector
Table 17 summarises the eight high risk issues associated with the abalone processing sector and the potential controls.
Significant risk issues related to the abalone processing sector
Table 18 summarises the six risk issues that were considered significant for the abalone processing sector. Appropriate controls are also summarised.
Moderate risk issues associated with the abalone processing sector
Three moderate risk issues were identified for the processing sector and are summarised in Table 19.
Table 17: High risk issues associated with the abalone processing sector
| Pathway | Issue | Controls |
|---|---|---|
| Processor to farm | Movement of live infected abalone from a processor to a farm in another/adjoining zone. Movement of live infected abalone from a processor to a farm in another state. | This applies to farms that source broodstock through processors. In Victoria, this is no longer permitted. In states where it is permitted broodstock should be subject to health certification. |
| Processor to wild | Transfer of virus to uninfected wild areas through inappropriate disposal of viscera, shells, mortalities and other processing wastes, including sale of by-products as bait to fishermen. | All processing wastes should be disposed of according to approved protocols. |
| Processor to processor | Movement of dead/ semi processed animals (abalone and other species that may harbour the virus) between processors - interstate. Movement of dead/ semi processed animals (abalone and other species that may harbour the virus) between processors - international. | Animals should be fully processed before movement from the processing facility. |
Movement of live infected animals (abalone and other species that may harbour the virus) between processors - interstate. Movement of live infected animals (abalone and other species that may harbour the virus) between processors - international. | Staff should be trained to identify infected animals. Infected animals should not be moved, but reported to authorities. | |
Movement of contaminated transport water may carry virus particles between processors. | Transport water should be disposed to the sewage system, which is disinfected on a regular basis. |
Table 18: Significant risk issues associated with the abalone processing sector
| Pathway | Issue | Controls |
|---|---|---|
| Processor to farm | Movement of live infected abalone from a processor to a farm - local movements within a zone. | No movement of live infected abalone permitted. Farms should not be permitted to source broodstock from processors. |
Movement of contaminated equipment and personnel introduces disease from a processor to a farm. | Bio-security protocols for aquaculture farms should be implemented to prevent disease transfer to farms. | |
| Processor to wild | Movement of live infected animals through processor for sea-ranching or seacage farming. | Infected animals should not be moved. Stocking of seacages and sea-ranching can only be undertaken with approval from Fisheries. |
| Processor to processor | Movement of live infected animals (abalone and other species that may harbour the virus) between processors - local movements. Movement of live infected animals (abalone and other species that may harbour the virus) between processors - adjoining zones. | Staff should be trained to identify signs of the disease. No movement of infected animals. |
Movement of contaminated equipment, vehicles and personnel introduces virus between processors. | Apply appropriate disinfection protocols. |
Table 19: Moderate risk issues associated with the processing sector
| Pathway | Issue | Controls |
|---|---|---|
| Processor to wild | Transfer of virus to uninfected wild areas through inappropriate disposal of process effluent water. Virus transferred to wild by contaminated equipment. | Contain and treat process effluent water. |
Processor to processor | Movement of dead/ semi processed animals (abalone and other species that may harbour the virus) between processors - adjoining zones. | Contain and treat process effluent water. |
Benefits and adoption
The outputs of the Risk Assessment Workshop were used to develop Codes of Practice for various sectors of the abalone industry. This document, "Biosecurity Control Measures for Abalone Viral Ganglioneuritis: A Code of Practice", has been published by Fisheries Victoria as a separate, stand-alone document3 so that it can be disseminated to stakeholders. Victoria was used as a case-study, but the key elements of the Code of Practice can be adapted for other states with an abalone industry.
All sectors of the abalone industry, including wild-capture, recreational, aquaculture and processing will directly benefit from the increased bio-security that will arise through the implementation of the Codes of Practice. For the Australian abalone aquaculture industry, the implementation of the Code of Practice and the necessary increased attention to aquatic bio- security will result in enhanced production by:
- minimising morbidity and mortality
- obtaining an optimal growth response
- ensuring high survival of stock
- preventing the spread of infectious disease.
In addition, the other Codes of Practice will assist in maintaining the favourable health status of Australia's wild fisheries through protocols that will lead to improved bio-security in the wild catch and processing sectors. All industry sectors will be better protected by being more prepared to respond to, control and manage the negative impacts associated with disease outbreaks.
Social and community benefits will flow from further protection of natural biodiversity and the security of both the recreational and commercial fishery for abalone that provides recreation, jobs and income for community members.
Further development
As outlined in the literature review, current knowledge on the virus is very limited. Future developments in this area need to concentrate on addressing the knowledge gaps identified in this report. In particular, pre-clinical diagnostic techniques must be developed to allow for identification of the virus in otherwise healthy animals. This will allow surveillance programs to be undertaken.
Conclusion
This project has developed Codes of Practice to increase bio-security in the Australian abalone industry and minimise the risk of outbreaks and spread of abalone viral ganglioneuritis. In developing these Codes of Practice, a robust and defensible process was undertaken to identify the key issues that needed to be addressed by the wild harvest, recreational, aquaculture and processing sectors. These issues were assessed and prioritised at a Risk Assessment Workshop by an expert panel assembled from all Australian states with an abalone industry. The panel included aquatic animal health specialists, representatives from industry and Government officials.
The Risk Assessment Workshop identified the most important issues for each industry sector and control measures were subsequently developed (bearing in mind that knowledge of the virus is incomplete). The controls were subsequently developed into a Code of Practice for each industry sector that is comprised of a series of Standard Operating Procedures.
References
ABARE. (2006). Australian Fisheries Statistics 2005. Australian Bureau of Agricultural and Resource Economics, Canberra. 68 pp.
Ahne, W. (1994). Viral infections of aquatic animals with special reference to Asian aquaculture. Annual Review of Fish Diseases 4: 375-388.
Alderman, D., J. (1980). Shellfish diseases: past, present, and future. In: Proc. 11th Ann. Conference. Shellfish Assoc. Great Britain. Pp 10-40.
Allen, S.K. (1998). Commercial applications of bivalve genetics: not a solo effort. World Aquaculture 29: 38-43.
Arzul, I., Nicolas, J.L., Davison, A.J., and Renault, T. (2001a). French scallops: A new host for ostreid herpesvirus-1. Virology 290(2): 342-349.
Arzul, I., and Renault, T. (2002). Herpesvirus et bivalves marins. Virologie 6: 169-174.
Arzul, I., Renault, T., and Lipart, C. (2001b). Experimental herpes-like viral infections in marine bivalves: demonstration of interspecies transmission. Diseases of Aquatic Organisms 46(1): 1-6.
Arzul, I., Renault, T., Lipart, C., and Davison, A.J. (2001c). Evidence for interspecies transmission of oyster herpesvirus in marine bivalves. Journal of General Virology 82(4): 865-870.
Arzul, I., Renault, T., Thebault, A., and Gerard, G. (2002). Detection of oyster herpesvirus DNA and proteins in asymptomatic Crassostrea gigas adults. Virus Research 84: 151-160.
Barbosa-Solomieu, V., Degremont, L., Vazquez- Juarez, R., Ascencio-Valle, F., Boudry, P., and Renault, T. (2005). Ostreid Herpesvirus 1 (OsHV-1) detection among three successive generations of Pacific oysters (Crassostrea gigas). Virus Research 107(1): 47-56.
Batista, F.M., Taris, N., Boudry, P., and Renault, T. (2005). Detection of ostreid herpesvirus-1 (OsHV-1) by PCR using a rapid and simple method of DNA extraction from oyster larvae. Diseases of Aquatic Organisms 64(1): 1-4.
Botes, H., Basson, L., and Van As, J.G. (1998). Ectosymbiotic ellobiophryid peritrichs associated with abalone. Proceedings of the Microscopy Society of Southern Africa 28(75.).
Bower, S.M. (1995). Synopsis of infectious diseases and parasites of commercially exploited shellfish: Withering syndrome of abalone.
Bower, S.M. (2000). Infectious diseases of abalone (Haliotis spp.) and risks associated with transplantation. Can. Spec. Publ. Fish. Aquat. Sci 130: 111-122.
Bower, S.M. (2001). Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish: Amyotrophia of Abalone. http://www-sci.pac.dfo- mpo.gc.ca/shelldis/amyotab_e.htm.
Bower, S.M. (2003). Update on emerging abalone diseases and techniques for health assessment. Journal of Shellfish Research 22(3): 805-810.
Bower, S.M., McGladdery, S.E., and Price, I.M. (1994). Synopsis of infectious diseases and parasites of commercially exploited shellfish. Annual Review of Fish Diseases 4: 1-199.
Chang, P.H., Kuo, S.T., Lai, S.H., Yang, H.S., Ting, Y.Y., Hsu, C.L., and Chen, H.C. (2005). Herpes-like virus infection causing mortality of cultured abalone Haliotis diversicolor supertexta in Taiwan. Diseases of Aquatic Organisms 65(1): 23-27.
Chiu Yu-Tzu. (2003). PFP lawmaker questions COA about abalone virus. Taipei Times, 13 March 2003, P 2
Comps, M., and Cochennect, N. (1993). A herpes- like virus from the European oyster Ostrea edulis L. Journal of Invertebrate Pathology 62(2): 201-203.
Cunningham, C.O. (2002). Molecular diagnosis of fish and shellfish diseases: Present status and potential use in disease control. Aquaculture 206(1-2): 19-55.
Davison, A.J. (2002). Evolution of the herpesviruses. Vet. Microbiol. 86: 69-88.
Davison, A.J., Trus, B.L., Cheng, N., Steven, A.C., Watson, M.S., Cunningham, C., Le Deuff, R.M., and Renault, T. (2005). A novel class of herpesvirus with bivalve hosts. Journal of General Virology 86(1): 41-53.
Dixon, M.G., Hecht, T., and Brandt, C.R. (1991). Identification and treatment of Clostridium and Vibrio infection in South African abalone, Haliotis midae L. Journal of Fish Diseases 14: 693-695.
Elston, R.A. (1984). Prevention and management of infectious diseases in intensive mollusc husbandry. Journal of the World Mariculture Society 15: 284-300.
Farley, C.A., Banfield, W.G., Kasnic, G., and Foster, W.S. (1972). Oyster herpes-type virus. Science 178(4062): 759-760.
Friedman, C.S. (1991). Coccidiosis of California abalone, Haliotis spp. Journal of Shellfish Research 10: 236.
Fuhrman, J.A. (1999). Marine viruses and their biogeochemical and ecological effects. Nature 399: 541-548.
Goggin, C.L., and Lester, R.J.G. (1995). Perkinsus, a protistan parasite of abalone in Australia: a review. Marine Fisheries Research 46: 639-646.
Gorfine, H.K. (2002). Assessment of the Sustainability of Victorian abalone Resources. PhD Thesis. University of Technology Sydney.
Handlinger, J., Bastianello, S., Callinan, R., Carson, J., Creeper, J., Deveney, M., Forsyth, W.M., Freeman, K., Hooper, C., Jones, B., Landcaster, M., Landos, M., Loh, R., Oyay, B., Phillips, P., Pyecroft, S., and Stephens, F. (2006). Abalone aquaculture subprogram: A national survey of diseases of commercially exploited abalone species to support trade and translocation issues and the development of health surveillance programs 2002/201. FRDC Project No. 2002/201. Fisheries Research and Development Corporation, Canberra. 170 pp.
Handlinger, J., Carson, J., Donachie, L., Gabor, L., and Taylor, D. (2005). Bacterial infection in Tasmanian farmed abalone: Causes, pathology, farm factors and control measures. In: P. Walker, R. Lester, and M.G. Bondad-Reantaso (Eds), Diseases in Asian Aquaculture V. Fish Health Section, Asian Fisheries Society, Manila. Pp 289- 299.
Hardy-Smith, P. (2006). Report on the events surrounding the disease outbreak affecting farmed and wild abalone in Victoria. Confidential report for the Department of Primary Industries Victoria. Panaquatic® Health Solutions Pty Ltd. 47 pp.
Hine, P.M. (1996). Southern hemisphere mollusc diseases and an overview of associated risk assessment problems. Rev. sci. tech. Off. int. Epiz. 15(2): 563-578.
Hine, P.M. (1997). Trends in research on diseases of bivalve molluscs. Bulletin of the European Association of Fish Pathologists 17: 181-183.
Hine, P.M., and Thorne, T. (1997). Replication of herpes-like viruses in haemocytes of adult flat oysters Ostrea angasi: An ultrastructural study. Diseases of Aquatic Organisms 29(3): 189-196.
Hine, P.M., Wesney, B., and Besant, P. (1998). Replication of a herpes-like virus in larvae of the flat oyster Tiostrea chilensis at ambient temperatures. Diseases of Aquatic Organisms 32(3): 161-171.
Hine, P.M., Wesney, B., and Hay, B.E. (1992). Herpesviruses associated with mortalities among hatchery-reared larval Pacific oysters Crassostrea gigas. Diseases of Aquatic Organisms 12(2): 135-142.
Huang, Y.Y., Wu, W.Z., Yan, J.H., Zhou, W.B., Chen, X.Z., Ni, Z.M., and X.T., C. (1999). Investigation on an exterminate disease of Haliotis diversicolor aquatiliss. Fujiau Journal of Animal Husbandry and Veterinary 21(3): 4-5.
ICES (2003). Report of the Working Group on Pathology and Diseases of Marine Organisms, Aberdeen, UK, 11-15 March 2003. International Council for the Exploration of the Sea, Copenhagen, Denmark. 95 pp.
ICES (2004). Report of the Working Group on Pathology and Diseases of Marine Organisms (WGPDO) (9-13 March 2004, Abo, Finland). International Council for the Exploration of the Sea, Copenhagen, Denmark. 80 pp.
ICES. (2005). Report of the Working Group on Pathology and Diseases of Marine Organisms (WGPDO) (8-12 March 2005, La Tremblade, France). International Council for the Exploration of the Sea, Copenhagen, Denmark. 106 pp.
ICES (2006). Report of the Working Group on Pathology and Diseases of Marine Organisms (WGPDO) (7-11 March 2006, ICES Headquarters, Copenhagen). International Council for the Exploration of the Sea, Copenhagen, Denmark. 98 pp.
Johnson, P.T. (1976). A herpes-like virus from the blue crab, Callinectes sapidus. Journal of Invertebrate Pathology 27: 419-420.
Jones, J.B.C.J. (2006). Diseases of pearl oysters and other molluscs: A Western Australian perspective. Journal of Shellfish Research 25(1): 233-238.
Le Deuff, R.M., Nicolas, J.L., Renault, T., and Cochennec, N. (1994). Experimental transmission of herpes-like virus to axenic larvae of Pacific oyster (Crassostrea gigas). Bulletin of the European Association of Fish Pathologists 14: 69-72.
Le Deuff, R.M., Renault, T., and Gerard, A. (1996). Effects of temperature on herpes- like virus detection among hatchery- reared larval Pacific oyster Crassostrea gigas. Diseases of Aquatic Organisms 24(2): 149-157.
Lees, D. (2000). Viruses and bivalve shellfish. International Journal of Food Microbiology 59(1-2): 81-116.
Li, X., Wang, B., and Li, S.-F. (1998). Studies on pathology and histopathology of ʺcrack shell diseaseʺ of Haliotis discus hannai. Journal of Fisheries of China 22(1): 61-66.
Lipart, C., and Renault, T. (2002). Herpes-like virus detection in infected Crassostrea gigas spat using DIG-labelled probes. Journal of Virological Methods 101(1-2): 1- 10.
Love, G., and Langenkamp, D. (2003). Australian aquaculture: Industry profiles for selected species ABARE eReport 03.8. Australian Bureau of Agricultural and Resource Economics, Canberra. 128 pp.
Ma, J.M., Wang, Q., Ma, F.H., and Liu, M.Q. (1996). A pathogen of septicopyaemia in the abalone Haliotis discus hannai. Dalian. China 20(4): 332-336.
McDiarmid, H., Day, R., and Wilson, R. (2004). The ecology of polychaetes that infest abalone shells in Victoria, Australia. Journal of Shellfish Research 23(4): 1179- 1188.
Meyers, T.R. (1984). Marine mollusks as reservoirs of viral finfish pathogens: significance to marine and anadromous finfish aquaculture. Mar. Fish. rev. 46: 14- 17.
Momoyama, K., Nakatsugawa, T., and Yurano, N. (1999). Mass mortalities of juvenile abalones, Haliotis spp., caused by amyotrophia. Fish Pathology 34(1): 7-14.
Mouton, A. (2000). Health management and disease surveillance in abalone, Haliotis midae, in South Africa. In: International Abalone Symposium, Cape Town, South Africa, February, 2000 (Journal of Shellfish Research 19(1) p. 526).
Muroga, K. (2001). Viral and bacterial diseases of marine fish and shellfish in Japanese hatcheries. Aquaculture 202(1-2): 23-44.
Naciri-Graven, Y., Martin, A.G., Baud, J.P., Renault, T., and Gerard, A. (1998). Selecting the flat oyster Ostrea edulis (L.) for survival when infected with the parasite Bonamia ostreae. Journal of Experimental Marine Biology & Ecology 224: 91-107.
Nakatsugawa, T., Nagai, T., Hiya, K., Nishizawa, T., and Muroga, K. (1999). A virus isolated from juvenile Japanese black abalone Nordotis discus discus affected with amyotrophia. Diseases of Aquatic Organisms 36(2): 159-161.
Nakatsugawa, T., Okabe, M., and Muroga, K. (2000). Horizontal transmission of amyotrophia in Japanese black abalone. Fish Pathology 35: 11-14.
Nell, J., and Perkins, B. (2006). Evaluation of the progeny of third-generation Sydney rock oyster Saccostrea glomerata (Gould, 1850) breeding lines for resistance to QX disease Marteilia sydneyi and winter mortality Bonamia roughleyi. Aquaculture Research 37: 693-700.
Nicolas, J.L., Comps, M., and Cochennec, N. (1992). Herpes-like virus infecting Pacific oyster larvae, Crassostrea gigas. Bulletin of the European Association of Fish Pathologists 12(1): 11.
Otsu, R., and Sasaki, K. (1997). Virus-like particles detected from juvenile abalones (Nordotis discus discus) reared with an epizootic fatal wasting disease. Journal of Invertebrate Pathology 70: 167-168.
Payen, G.G., and Bonami, J.R. (1979). Mise en evidence de particules dʹallure virale associees aux noyaux des cellules mesodermiques de la zone germinative testiculaire du crabe Rhithropanopeus harrisii (Gould). Rev. Trav. Inst. Pech. Marit 43: 361-365.
Renault, T. (1996). Appearance and spread of diseases among bivalve molluscs in the northern hemisphere in relation to international trade. Rev. sci. tech. Off. int. Epiz. 15(2): 551-561.
Renault, T. (2003). Annex 11: Obtain information on the EU Project ʺDiagnosis of oyster herpes-like virus: development and validation of molecular, immunological and cellular toolsʺ (FAIR-PL98-4334) and review the results. In: WGPDMO (Ed) Report of the Working Group on Pathology and Diseases of Marine Organisms, Aberdeen, UK, 11-15 March 2003. International Council for the Exploration of the Sea, Copenhagen, Denmark. Pp 61-65.
Renault, T., and Arzul, I. (2001). Herpes-like virus infections in hatchery-reared bivalve larvae in Europe: Specific viral DNA detection by PCR. Journal of Fish Diseases 24(3): 161-167.
Renault, T., Arzul, I., and Lipart, C. (2004). Development and use of an internal standard for oyster herpesvirus 1 detection by PCR. Journal of Virological Methods 121(1): 17-23.
Renault, T., Cochennec, N., LeDeuff, M., and Chollet, B. (1994a). Herpes-like virus infecting Japanese oyster (Crassostrea gigas) spat. Bulletin of the European Association of Fish Pathologists 14(2): 64.
Renault, T., Le Deuff, R.M., Chollet, B., Cochennec, N., and Gerard, A. (2000a). Concomitant herpes-like virus infections in hatchery-reared larvae and nursery- cultured spat Crassostrea gigas and Ostrea edulis. Diseases of Aquatic Organisms 42(3): 173-183.
Renault, T., Le Deuff, R.M., Cochennec, N., and Maffart, P. (1994b). Herpesvirusassociated with mortalities among Pacific oyster, Crassostrea gigas, in France - comparative study. Revue Medecine Veterinaire 145(10): 735-742.
Renault, T., Le Deuff, R.M., Lipart, C., and Delsert, C. (2000b). Development of a PCR procedure for the detection of a herpes-like virus infecting oysters in France. Journal of Virological Methods 88(1): 41-50.
Renault, T., Lipart, C., and Arzul, I. (2001a). A herpes-like virus infecting Crassostrea gigas and Ruditapes philippinarum larvae in France. Journal of Fish Diseases 24(6): 369-376.
Renault, T., Lipart, C., and Arzul, I. (2001b). A herpes-like virus infects a non-ostreid bivalve species: virus replication in Ruditapes philippinarum larvae. Diseases of Aquatic Organisms 45(1): 1-7.
Renault, T., and Novoa, B. (2004). Viruses infecting bivalve molluscs. Aquatic Living Resources 17(4): 397-409.
Reuter, R.E., and McOrist, S. (1999). Mortality due to Vibrio harveyi in farmed blacklip abalone, Notohaliotis ruber. In: Book of Abstracts. The Annual International Conference and Exposition of the World Aquaculture Society, 26April - 2 May 1999, Sydney, Australia. World Aquaculture Society. P. 629.
Roizman, B., and Baines, J. (1991). The diversity and unity of Herpesviridae. Comprehensive Immunology and Microbiology in Infectious Diseases 14(2): 63-79.
Shi Zhengli, and Handlinger, J. (2004). Abalone viral mortality -disease card. Developed to support the NACA/FAO/OIE regional quarterly aquatic disease (QAAD) reporting system in the Asia-Pacific. NACA, Bangkok. 5 pp.
Song, Z.H.R., Ji, R.X., Yan, S.F., and Chen, C.S. (2000). A spherovirus resulted in mass mortality of Haliotis diversicolor aquatilis. Journal of Fisheries of China 24(5): 463-467.
Sparks, A.K., and Morado, J.F. (1986). A herpes- like virus disease in the blue king crab Paralithodes platypus. Diseases of Aquatic Organisms 1(2): 115-122.
Sparks, A.K., and Morado, J.F. (1997). Some diseases of northeastern Pacific commercial crabs. Journal of Shellfish Research 16(1): 320.
Sumner, J., and Ross, T. (2002). A semi- quantitative seafood safety risk assessment. International Journal of Food Microbiology 77(1-2): 55-59.
Wang, B., and Li, X. (1997). Infection of spherical viruses from Haliotis discus hainai. Ino. Virologica Sinica 12(4): 360-363.
Wang, J., Guo, Z., Feng, J., Liu, G., Xu, L., Chen, B., and Pan, J. (2004). Virus infection in cultured abalone, Haliotis diversicolor Reeve in Guangdong Province, China. Journal of Shellfish Research 23(4): 1163- 1168.
Wang, J.Y., Chen, B.S.H., Feng, J., and Yu, M.Y. (2000). Primary observations of spherical viruses from diversicolor abalone (Haliotis diversicolor Reeve). Journal of Tropical Oceanography 12: 63-67.
Ye, L., Yu, K.K., and Wang, R.C. (1997). The study on the pathogenic bacteria of the fester disease of cultured juvenile discus abalone. Journal of Fishery Sciences China 4(4): 43-48.
Zhang, Z.H.X., Wang, J., Su, Y.Q., Yan, Q.P., Chi, X.C., Zhou, H.M., and Zhou, Y.C. (2001). Pathogeny and histopathology of the epidemic disease in Haliotis diversicolor supertexta. Journal of Xiamen University 40(4): 949-956.
Appendix I: Summary of diseases and parasites reported from abalone
For further details and related references see Bower et al. 1994; Hine 1996; Bower 2000; 2003; Handlinger et al. 2006; Jones 2006 and pac.dfo-mpo.gc.ca/sci/shelldis/title_e.htm)
| Disease/parasite | Species | Location | Source | Size | Comments |
|---|---|---|---|---|---|
| Viruses | |||||
| Herpes-like virus disease | H. diversicolor supertexta | Taiwan | Cultured & wild | Juveniles & adults | Serious disease causing mass mortality (Chang et al. 2005) |
| Spherical Virus | H. diversicolor supertexta H. discus hannai | China | Cultured | All sizes | Serious disease causing high mortalities. Associated with "crack-shell disease". Causes necrosis and disorders of connective tissues of all organs (see Wang and Li 1997; Li et al. 1998; Huang et al. 1999; Song et al. 2000; Wang et al. 2000; Zhang et al. 2001; Shi Zhengli and Handlinger 2004; Wang et al. 2004) |
| Infectious amyotrophia (Virus-like particles) | H. discus discus H. discus hannai Nordotis madaka | Japan | Cultured | Juveniles | Serious disease causing muscle atrophy in the mantle and foot resulting from tumours of the pedal ganglia and nerve trunks which impedes feeding and adhesion to the substrate, followed by impaired shell growth and mortality. Agent is unknown, but retrovirus-like particles were observed. Epizootic mass mortalities attributable to this disease have been observed. Usually occurs at 18 to 20 °C (see Otsu and Sasaki 1997; Momoyama et al. 1999; Nakatsugawa et al. 1999; Nakatsugawa et al. 2000) |
| Intestinal viral inclusions I - GIT | H. rubra | Australia (Tas & WA) | Cultured & wild | Putative viral infection (Handlinger et al. 2006) | |
| Intestinal viral inclusions II - Granulocytes | Australia (Tas) | Wild | Putative viral infection (Handlinger et al. 2006) | ||
| Eubacteria | |||||
| Rickettsia-like prokaryote, including "Candidatus Xenohaliotis californiensis" (withering disease) | H. midae H. fulgens H. cracherodii H. rufescens H. corrugata H. sorenseni H tuberculata | South Africa, Mexico, USA & Chile (Japan, Israel, Iceland Ireland, China) | Cultured & wild | All sizes | Severe disease causing mass mortality. Withering disease is reportable to the OIE. Intracellular infection that causes lethargy, retracted visceral tissues and atrophy of the foot muscle. Degradation of the tubules in the digestive gland results in starvation and ultimately withering of the muscle. Elevated temperatures accelerate disease progression and decrease survival. |
| Rickettsia-like prokaryote | H. rubra, H. laevigata | Australia (Tas, SA, NSW & WA) | Cultured & wild | Dissimilar to withering disease | |
| Bacteria | |||||
| Vibrio carchariae (syn V. harveyi?) | H. diversicolor supertexta H. tuberculata | Japan & France | Cultured & wild | High mortalities. Associated with white spots, white necrotic muscle fibres and bacteria on the foot. | |
| H. rubra H. laevigata | Australia (Vic, Tas. WA & SA) | Cultured | Abscess-like formations and septicaemia. Stress related. H. rubra is very susceptible. | ||
| V. carchariae (syn V. harveyi?) | H. rubra H. laevigata | Australia (Vic, Tas. WA & SA) | Cultured | Abscess-like formations and septicaemia. Stress related. H. rubra is very susceptible. | |
| V. splendidus I | H. rubra | Australia (Tas) | Cultured & wild | ||
| V. alginolyticus | H. midae H. rufescens | South Africa, USA & Tawain | Cultured | Larvae & juveniles | Associated with stressful conditions (see Dixon et al. 1991) |
| V. campbellii | H. discus hannai | Septicopyaemia (see Ma et al. 1996) | |||
| V. fluvialis-II (pustule disease or Blister disease) | H. discus hannai | China | Cultured | Various growth stages | Severe disease and mortality. Blister-like lesions on the foot. Resistant to 18 different antibiotics. |
| V. parahaemolyticus | Tawain | Juveniles | Causes gastroenteritis in humans | ||
| Tenacibaculum maritimum (syn. Flexibacter maritimus) | Australia | ||||
| Flavobacterium-like bacteria (Flavobacterial syndrome) | H. laevigata H. rubra | Australia | Cultured & wild | Stress related | |
| Clostridium lituseberense | H. midae | South Africa | Laborato ry | Juveniles | Associated with stressful conditions (see Dixon et al. 1991) |
| Pseudomonas fluoresces | H. discus hannai | Cultured | Juveniles | Fester disease (see Ye et al. 1997) | |
| Bacteria- general | H. rufescens H. kamtschatkana H. rubra H. laevigata H. tuberculata, H. midae | Global | Mainly cultured | Mainly juveniles | Systemic infection of the soft-tissues resulting in tissue necrosis and death. |
| Funga | |||||
| Fungal diseases (foot tubercles and shell mycosis), including Atkinsiella awabbi and Haliphthoros milfordensis | H. sieboldii H. iris H. australis H. virginea H. rubra H. laevigata | Japan, New Zealand & Australia (several states) | Cultured & wild | Tubercle mycosis involves tubercle-like swellings on the mantle and foot. Shell mycosis involves blisters of conchiolin and occasionally nacreous material on the inner shell surface near the shell apex. | |
| Protozoa | |||||
| Haplosporidian | H. iris | New Zealand | Cultured & wild | Juveniles & adults | Causes chronic mortalities being most severe during summer and early autumn when water temperatures high (at 21°C) |
| Perkinsus spp., including P. olseni (Perkinsosis) | H. rubra H. laevigata H. cyclobates H. scalaris | Australia (SA, WA, NSW, Vic & Qld) | Cultured & wild | Severe disease causing mortalities. Perkinsosis is reportable to the OIE. Proliferates in tissues and may produce pustules in the foot and mantle. Infections possibly correlated with water temperature & abalone size. | |
| Labyrinthuloides haliotidis | H. kamtschatkana H. rufescens | Canada | Cultured | juveniles | Severe disease causing destruction of head and foot tissues and subsequent mortality of up to 100%. |
| Coccidian and coccidian-like parasites, including Pseudoklossia (syn - Margolisiella ) - type coccidia (Abalone kidney coccidia) (i.e. P. haliotis) | H. cracherodii H. rufescens H. corrugata H. fulgens H. walallensis H. kamtschatkana H. midae H. iris H. roei | South Africa, USA, Canada, New Zealand, Mexico & Australia (Tas & WA) | Cultured & wild | Infected kidney epithelial cells become extremely hypertrophied and heavy infections appear to cause serious kidney damage. In some cases associated with stress (see Friedman 1991; Mouton 2000). | |
| Ciliates (including species from the orders Thigmotrichida, Peritrichida, Heterotrichida and Hypotrichida) | H. laevigata H. rubra H. conicopora H. roei H. midae H. spadicea H. kamtschatkana H. rufescens Other species | Probably ubiquito us. South Africa, Canada, USA, Mexico & Australia (Vic, Tas, WA, NSW & SA) | Cultured & wild | Found attached to the gills, mantle cavity or oesophageal pouch epithelium. Many species may be commensal symbionts since large numbers elicit no obvious host-response. (Botes et al. 1998). | |
| Cryptosporidian- like parasites | Australia (Tas, WA & SA) | Cultured & wild | |||
| Apicomplexans | H. laevigata H. rubra | Australia (Tas) | Wild | ||
| Gregarines (including Nematopsis-like gregarines) | H. laevigata H. rubra | Australia (NSW, Tas & WA) | Cultured & wild | ||
| Polychaeta | |||||
| Sabellid polychaetes (including Terebrasabella heterouncinata) | H. midae H. rufescens H. fulgens H. corrugata | South Africa, USA, Iceland & Mexico | Cultured | Severe disease capable of causing reduced growth rates, deformed shells, decreased meat yields, reduced productivity and mortality. Sometimes associated with high stocking densities, poor hygiene and marginal water quality. | |
| Spionid polychaetes (including Boccardia knoxi B. chilensis, Polydora hoplura, P. haswelli, P. websterii, P. woodwicki Dipolydora armata & Boccardiella MoV 3480) | H. Rubra H. laevigata H. conicopora H. roei H. kamtschatkana H. diversicolor H. midae Other species | Global. Japan, South Africa, Canada & Australia (Tas, NSW, SA, Vic & WA) | Cultured & wild | Most infestations are innocuous, but can cause stress, flesh weight loss shell damage and blistering, and mortality in some cases (see McDiarmid et al. 2004). | |
| Molluscs | |||||
| Shell boring clams (including Lithophaga aristate, L. plumule & Penitella conradi) | H. fulgens H. corrugata | Mexico | Detrimental under adverse conditions | ||
| Others | |||||
| Cestodes | H. laevigata H. roei | Australia (WA) | Wild | Including plerocerci (metacestodes) | |
| Trematodes. Various species of Digenea (including species in the family Allocreadiidae and possibly Opeocoilidae) | H. roei H. rubra H. laevigata H. asinina H. tuberculata H. spadicea | South Africa & Australia (Vic. SA. NSW & WA) | Cultured & wild | Including metacercaria. Infesting (encysting) various organs and tissues including the foot, digestive gland, gill filaments and gonad. Prevalence and intensity of infection are low in most cases. Does not appear to affect survival. | |
| Nematodes (including Echinocephalus pseudouncinatus) | H. corrugata H. fulgens | USA, Mexico & Australia (SA) | Wild | Abalone may be intermediate host. Tissue damage of foot associated with burrowing of larval stages prior to encysting. | |
| Boring sponges | Australia (Tas & NSW) | ||||
Appendix II: List of Participants
| Participant | Affiliation/address | State |
|---|---|---|
| Dr Mehdi Doroudi | Director, Marine and Freshwater Systems, PIRVic, Queenscliff | VIC |
| Mr Anthony Forster | Manager, Aquaculture, Fisheries Victoria, Melbourne | VIC |
| Dr Brett Ingram | Senior Scientist, Marine and Freshwater Systems, PIRVic, Snobs Creek | VIC |
| Mr Peter Lawson | Senior Fisheries Management Officer, Fisheries Victoria, Geelong | VIC |
| Ms Caroline McGowan | PA to Medhi Doroudi | VIC |
| Mr Harry Peeters | Executive Officer, WADA | VIC |
| Mr Vin Gannon | Victorian Abalone Divers Association (VADA) | VIC |
| Mr David Tonkin | Executive Officer, Victorian Abalone Processors Association Inc. (VAPA) | VIC |
| Dr Paul Hardy-Smith | Managing Director, Panaquatic Health Solutions | VIC |
| Mr Stephen Rodis | Victorian Abalone Growers Association (VAGA) | VIC |
| Dr Judith Handlinger | Senior Veterinary Pathologist (Aquatic Animals), Fish Health Unit, Animal Health Laboratory, DPIW, Tasmania. | TAS |
| Dr Fran Stephens | Principal Fish Pathologist, Department of Fisheries, WA | WA |
| Dr Marty Deveny | Aquatic Health Officer, PIRSA Aquaculture, SA. | SA |
| Ms Jane Frances | A/ Manager Aquatic Bio-security & Risk Management, Port Stephens Fisheries Centre DPI, NSW | NSW |
| Dr Jacquelle Gorski | EPA Victoria | VIC |
| Dr Kevin Ellard | Senior Veterinary Officer, Aquatic Health, Dept Primary Industry and Water and Environment, Animal Disease Control DPIW, TAS | TAS |
| Mr Michael Tokley | Executive Officer, Australian Abalone Council (AAC) and Abalone Industry Association of SA Inc. | SA |
| Mr Alan Gray | Tasmanian Abalone Council | TAS |
| Mr Mark Jervis | Abalone Aquaculture Subprogram of FRDC | VIC |
| Dr Roger Paskin | Principal Veterinary Officer, DPI Attwood | VIC |
| Mr Stewart Mc Glashan | Fisheries Victoria | VIC |
Appendix III: Risk analysis process
Risk analysis is a key step in the development of Codes of Practice for the abalone industry sectors. It allows an objective appraisal of individual risks: it puts into context the likelihood that they will occur and the consequences if they do. Prior to the workshop a preliminary list of issues (Appendix IV) was compiled by the project team. It was based on the pathways and vehicles of transmission discussed earlier.
Experts in fish health and bio-security from across Australia participated in the workshop. Participants were drawn from both Government (State and Federal) and industry and are listed in Appendix II. Workshop participants reviewed the list of issues. They identified gaps and then assessed the likelihood and consequences of the identified risks using the ranking system described below.
Risk Ratings
The risk assessment process used at the workshop was based on the Australian/New Zealand Standard for Risk Management (AS/NZ 4360:2004). However, the definitions of "likelihood" and "consequence" were tailored to align with the overarching project objective, which was:
With this objective in mind, "likelihood" and "consequence" rankings were defined as follows:
Likelihood is defined as a general description of probability or frequency of an event occurring. In the case of transmission of abalone viral ganglioneuritis, there are two ways in which likelihood can be interpreted:
- Likelihood that the virus can be transmitted via that pathway and specific risk. In a worst-case scenario with no controls present, it is assumed that the event (virus transmission) could lead to clinical disease at the destination; OR
- Likelihood that the specific risk will lead to the establishment of disease in a previously non-infected population of abalone. In this scenario the likelihood of the event (disease establishment) occurring would depend on many other factors (such as virus being transmitted in sufficient quantity to establish infection; exposure of non-infected abalone to sufficient quantities of the virus to become infected etc.). In most cases there is inadequate information to adequately assess the likelihood of disease establishment.
For the purposes of this risk assessment "likelihood" has been defined as the former, i.e. the likelihood that the virus can be transmitted via that pathway and specific risk. It is further assumed that exposure to the virus will result in establishment of the disease so that the worst-case scenario could be assessed. for likelihood are shown below:
Likelihood risk scores
| Score | Definition | |
|---|---|---|
| 1 | Rare | Chances of event occurring so small that it can be ignored in practical terms. |
| 2 | Unlikely | Event would be unlikely to occur. |
| 3 | Moderate | Event should occur at some time (e.g. once every few years). |
| 4 | Likely | Event likely to occur periodically (e.g. once per year). |
| 5 | Almost certain | Event would be expected to occur frequently (e.g.. Several times per year) |
Consequence is defined as the outcome or impact of an event, in this case the transmission of the virus via a pathway or specific risk. Given that the overarching objective of the project is to protect the Australian abalone, it was decided that consequence rankings should reflect the impact on the industry as a whole rather than the impact on an individual site, farm or processor. That is, although the consequences to the site, farm or processor of abalone viral ganglioneuritis would be catastrophic to the individual, it is the impact on the entire industry that needs to be assessed. As outlined earlier, it was assumed that exposure to the virus would result in establishment of the disease so that the worst- case scenario could be assessed.
Scores for consequences are shown below:
Likelihood risk scores
| Score | Definition | |
|---|---|---|
| 1 | Insignificant | No serious consequences to either wild or farm stocks.. |
| 2 | Minor | Localised disease outbreak restricted to specific area (e.g. reef) or single farm or single processor (local consequences). |
| 3 | Moderate | Disease outbreak in several adjacent reefs or several adjacent farms or several adjacent processors (regional consequences). |
| 4 | Major | Disease outbreak spread across multiple zones within a state (state-wide consequences). |
| 5 | Catastrophic | Disease outbreak spread across states and internationally (interstate/international consequences). |
Workshop participants were split into 3 groups and asked to assess the risks associated with the three sectors individually. All risks were rated under the assumption that the virus was present. The risk rankings were calculated by multiplying the likelihood score and the consequence score to give rankings from low to high.
| Likelihood | |||||
|---|---|---|---|---|---|
| 5 | 5 | 10 | 15 | 20 | 25 |
| 4 | 4 | 8 | 12 | 16 | 20 |
| 3 | 3 | 6 | 9 | 12 | 15 |
| 2 | 2 | 4 | 6 | 8 | 10 |
| 1 | 1 | 2 | 3 | 4 | 5 |
| Consequence | 1 | 2 | 3 | 4 | 5 |
| Risk Ratings | ||
|---|---|---|
| 1 | 4 | Low |
| 5 | 10 | Moderate |
| 12 | 15 | Significant |
| 16 | 25 | High |
Appendix IV: Risk ratings associated with spread of abalone viral ganglioneuritis.
Pathway 1: Movement within the wild.
| Pathway | No. | Specific Risk | Likelihood | Consequence | Rating | Comments |
|---|---|---|---|---|---|---|
| P1 | 1 | Natural movement of live infected animals (all life stages) between reefs/zones. | 3 | 4 | 12 | Will depend on distance between reefs. Assumes infected abalone have the capability of crawling between reef systems and the disease is vertically transmitted through settling spat. |
| P1 | 2 | Deliberate movement of live infected animals between reefs (recreational activities, restocking/re-seeding programs & bioterrorism). | 2 | 4 | 8 | Movement of undersized abalone, reef restocking/re-seeding activities and possibly bioterrorism. Is there currently deliberate movement of stock? |
| P1 | 3 | Movement of contaminated equipment and personnel by commercial divers from infected reef/zone to un- infected reefs/zones. | 5 | 5 | 25 | e.g. There will be enough viable virus surviving on equipment and personnel in the trip from one zone to infect and cause disease in abalone populations at another zone/site. |
| P1 | 4 | Movement of contaminated equipment and personnel by other human activities (recreational diving, recreational angling etc.) from infected reef/zone to an un- infected reefs/zones. | 5 | 5 | 25 | e.g. Recreational divers, illegal fishing (abalone poaching), non- abalone commercial fisheries, recreational angling, research activities, Fisheries officers inspecting multiple boats. High risk regardless as to whether there is a control measures in place. One example may be recreational divers catching animals at one reef and shucking and disposing of wastes at another reef. |
| P1 | 5 | Movement of virus contaminated water between reefs/zones (transfer of virus particles in current). | 5 | 5 | 25 | Passive movement in water currents and water vessel tank/bilge/ballast water. Virus can be transferred through water column. Distance between reefs/zones an important factor. Caught abalone are cooled on boats and water runs off to the sea. Assumes that even with considerable dilution factors experienced in open ocean, enough viable virus will travel through the water from one reef to a neighbouring reef and establish infection. |
| P1 | 6 | Sea-ranching. Deliberate movement of live infected animals between reefs for sea- ranging purposes. | 5 | 5 | 25 | |
| P1 | 7 | Movement of the virus from infected reef/zone to an un- infected reefs/zones by other animals (carriers, vectors, predators, scavengers etc.). | 4 | 5 | 20 | Do other animals act as reservoirs or carriers of the virus? |
Pathway 2: Movement from the wild to abalone farms.
| Pathway | No. | Specific Risk | Likelihood | Consequence | Rating | Comments |
|---|---|---|---|---|---|---|
| P2 | 1 | Movement of live infected animals from an infected reef to an adjacent farm - local movements. | 3 | 3 | 9 | Mainly adult animals. |
| P2 | 2 | Movement of live infected animals from an infected reef to a farm in an adjoining zone in Victoria. | 3 | 4 | 12 | Mainly adult animals. |
| P2 | 3 | Movement of live infected animals from Victorian wild to interstate farm. | 3.0 | 3 | 9 | Mainly adult animals. |
| P2 | 4 | Movement of live infected wild animals to a Victorian farm from interstate. | 2 | 3 | 6 | |
| P2 | 5 | Movement (export) of live infected animals from Victorian wild to international farm. | 1 | 0 | ||
| P2 | 6 | Movement of equipment and farm personnel between farms. | 5 | 4 | 20 | e.g. recreational diving, sharing/storage of diving equipment, divers servicing farm water intakes, broodstock collection, visitors to farms etc. |
| P2 | 7 | Use of abalone feeds contaminated with virus. | 2 | 2 | 4 | e.g. Use of feeds that may contain infected abalone by- products or wastes. Inappropriate processing does not kill the virus. |
| P2 | 8 | Inlet water may carry virus particles from infected reef to farm. | 5 | 3 | 15 | Consequence to the farm is major. Assumes that enough viable virus particles are in the water column to withstand the considerable dilution factors. This is also influenced by how far the reef is from the inlet pipes of the farm, the currents in the area and particularly on the density of wild infected abalone on the reef system. |
| P2 | 9 | Introduction of other animal species that may harbour the virus from an infected reef/area onto a farm. | 2 | 3 | 6 | e.g. Holding of non-target species such as other shellfish species that may be vectors (one group assumed mussels were not a vector), as well as predators/scavengers etc (rats, birds). Assumes that the virus will either survive passage through the gastro-intestinal tract of another animal species, will survive on the outside of another animal species or will actually infect other species. |
Pathway 3: Movement from the wild to processors.
| Pathway | No. | Specific Risk | Likelihood | Consequence | Rating | Comments |
|---|---|---|---|---|---|---|
| P3 | 1 | Movement of live infected animals (abalone and other species that may harbour the virus) from wild to processor - local movements. | 5 | 2 | 10 | Different risks between domestic processors and live exporters. Combine risks 1, 2 and 3. Note that consequences are significantly influenced by whether or not processor handles live abalone or is a cannery. |
| P3 | 2 | Movement of live infected animals (abalone and other species that may harbour the virus) from wild to processor - regional movements. | 5 | 2 | 10 | |
| P3 | 3 | Movement of live infected animals (abalone and other species that may harbour the virus) from wild to processor - interstate movements. | 5 | 2 | 10 | |
| P3 | 4 | Movement of live infected animals (abalone and other species that may harbour the virus) from wild to processor - international movements. | 0 | 0 | 1 | AQIS export/import regulations - potential issue for transmission if exporting overseas |
| P3 | 5 | Movement of abalone transport containers from wild to processor. | 5 | 2 | 10 | e.g. Transport boxes/crates. Assume that this virus will not survive long-term ʺunprotectedʺ outside the host (protection = mucus, in animal). Can only introduce disease if you have live abalone that can become 'diseased'. Alternatively you could have abalone showing no signs but carrying the virus 'break' with disease at the processing plant. This would depend on the abalone being held for a period of time at the processor. |
| P3 | 6 | Movement of contaminated personnel and vehicles to processor. | 2 | 2 | 4 | |
| P3 | 7 | Movement of marine water from the environment may carry virus particles into processor. | 2 | 2 | 4 | e.g. Transport water, process water derived from marine environment. Freshwater only used in processors therefore low risk. However, holding facilities use marine water from the environment. If located close to infected area and this water is used for holding etc, then risk much higher. Some processors use fresh seawater if near sea. If not, live abalone kept at processor cannot introduce 'disease'. Can though cause the processing facility to become contaminated with virus and this may therefore act as a source of virus. |
| P3 | 8 | Introduction into processors of other species that may harbour the virus. | 2 | 2 | 4 | e.g. Other shellfish. What other local species might be processed? Abalone from NZ; Trochus from north Australia; seacucumber (frozen) northern Aus/Pacific; fresh rock lobster (others may include fouling organisms?). Raw or part processed abalone from overseas imported for processing. Are some of these movements affected by translocation policies and AQIS? |
Pathway 4: Movement from abalone farms to processors.
| Pathway | No. | Specific Risk | Likelihood | Consequence | Rating | Comments |
|---|---|---|---|---|---|---|
| P4 | 1 | Movement of live infected farmed animals from farm to live/holding processor - local movement. | 3 | 3 | 9 | Consequence depends on discharge of processor. This risk rating was split to ʺlive/holding processorʺ and ʺCannery processorʺ for many specific risks within this Pathway. |
| P4 | 2 | Movement of live infected farmed animals from farm to cannery processor - local movement. | 3 | 1 | 3 | Consequence depends on discharge of processor. |
| P4 | 3 | Movement of live infected farmed animals from farm to live/holding processor - regional movement. | 3 | 4 | 12 | |
| P4 | 4 | Movement of live infected farmed animals from farm to cannery processor - regional movement. | 3 | 1 | 3 | |
| P4 | 5 | Movement of live infected farmed animals from Victorian farm to live/holding processor - interstate movement. | 3 | 4 | 12 | Movement from interstate to Victorian processors was considered but scored low on assumption that disease not yet recorded outside Victoria. |
| P4 | 6 | Movement of live infected farmed animals from Victorian farm to cannery processor - interstate movement. | 3 | 1 | 3 | |
| P4 | 7 | Movement of live infected farmed animals from farm to processor - international movement. | 1 | 2 | 2 | AQIS export/import regulations. Currently no live export to international processors. |
| P4 | 8 | Movement of contaminated equipment and personnel from farm to processor. | 2 | 3 | 6 | e.g. Transport boxes/crates. |
| P4 | 9 | Transport water may carry virus particles into processor. | 2 | 2 | 4 | |
| P4 | 10 | Introduction into processor of other species that may harbour the virus. | 1 | 3 | 3 |
Pathway 5: Movement from abalone farms to wild sector.
| Pathway | No. | Specific Risk | Likelihood | Consequence | Rating | Comments |
|---|---|---|---|---|---|---|
| P5 | 1 | Unintentional movement of live infected farmed animals from farm to wild through escape of farmed stock. | 4 | 3 | 12 | |
| P5 | 2 | Intentional movement of live infected farmed animals to wild in same zone. | 4 | 3 | 12 | e.g. Sea-cage farming, ranching or stock enhancement/replenishment. |
| P5 | 3 | Intentional movement of live infected farmed animals to wild in another zone. | 4 | 4 | 16 | e.g. Sea-cage farming, ranching or stock enhancement/replenishment. |
| P5 | 4 | Intentional movement of live infected farmed animals to wild in other states. | 4 | 5 | 20 | e.g. Sea-cage farming, ranching or stock enhancement/replenishment. |
| P5 | 5 | Movement of contaminated equipment and farm personnel introduces disease into wild. | 4 | 3 | 12 | |
| P5 | 6 | Discharge of virus infected effluent water may transfer disease to adjacent reefs. | 4 | 3 | 12 | Depends on virus concentration, dilution factors and survival time of virus outside host. Group discussion indicated that likelihood should be 5 since water flow is all year round. |
| P5 | 7 | Farms may transfer the virus to uninfected wild areas through inappropriate disposal of viscera, shells and mortalities. | 4 | 3 | 12 | |
| P5 | 8 | Transfer of virus from a farm into wild by other animals that may harbour the virus (e.g. predators/ scavengers) | 4 | 3 | 12 |
Pathway 6: Movement from processors to aquaculture farms.
| Pathway | No. | Specific Risk | Likelihood | Consequence | Rating | Comments |
|---|---|---|---|---|---|---|
| P6 | 1 | Movement of live infected abalone from a processor to a farm - local movements within a zone. | 5 | 3 | 15 | e.g. Potential purchase of broodstock. May be affected by distance or extent of movement? |
| P6 | 2 | Movement of live infected abalone from a processor to a farm in another/adjoining zone. | 5 | 4 | 20 | |
| P6 | 3 | Movement of live infected abalone from a processor to a farm interstate. | 5 | 5 | 25 | |
| P6 | 4 | Movement of live infected abalone from a processor to international farm. | 1 | 1 | 1 | AQIS import/export regulations |
| P6 | 5 | Movement of contaminated equipment and personnel introduces disease from a processor to a farm. | 4 | 3 | 12 | May need to be broken down to geographic zones. |
| P6 | 6 | Introduction of other species that may harbour the virus from a processor to a farm. | 1 | 1 | 1 |
Pathway 7: Movement within and between abalone farms.
| Pathway | No. | Specific Risk | Likelihood | Consequence | Rating | Comments |
|---|---|---|---|---|---|---|
| P7 | 1 | Movement of live infected farmed abalone between areas within the farm. | 5 | 2 | 10 | |
| P7 | 2 | Movement of live infected farmed abalone between sea- cages-local movements | 5 | 3 | 15 | |
| P7 | 3 | Movement of live infected abalone between farms-local movements. | 5 | 3 | 15 | |
| P7 | 4 | Movement of live infected abalone between farms - adjoining zones within state. | 5 | 4 | 20 | May not be different with local. |
| P7 | 5 | Movement of live infected farmed abalone between seacages-adjoining zones within state. | 5 | 4 | 20 | May not be different with local. |
| P7 | 6 | Movement of live infected abalone between farms - interstate. | 5 | 5 | 25 | |
| P7 | 7 | Movement of live infected farmed abalone between seacages -interstate. | 5 | 5 | 25 | |
| P7 | 8 | Movement of live infected abalone between farms- international. | 5 | 5 | 25 | AQIS export/import regulations. |
| P7 | 9 | Movement of live infected farmed abalone between seacages-international. | 5 | 5 | 25 | AQIS export/import regulations. |
| P7 | 10 | Movement of contaminated equipment and personnel introduces virus to other parts of farm. | 4 | 2 | 8 | |
| P7 | 11 | Movement of contaminated equipment and personnel transfers virus between seacages. | 3 | 3 | 9 | |
| P7 | 12 | Movement of contaminated equipment and personnel transfers virus between farms. | 3 | 3 | 9 | |
| P7 | 13 | Movement of water may carry virus particles within farms. | 3 | 2 | 6 | |
| P7 | 14 | Movement of water may carry virus particles between farms. | 3 | 3 | 9 | Depends on distance between farms (further apart will be 2 or 1). |
| P7 | 15 | Movement within farm of other species that may harbour the virus. | 3 | 2 | 6 | e.g.. Vermin, predators, scavengers, snails, dog walking through water, birds, rats other vectors etc. |
| P7 | 16 | Movement between seacages of other species that may harbour the virus. | 3 | 2 | 6 | Depends on distance between farms (further apart will be 2 or 1). |
| P7 | 17 | Movement between seacages of other species that may harbour the virus. | 2 | 3 | 6 | Depends on distance sea-cages (further apart will be 2 or 1) |
Pathway 8: Movement from processors to wild sector.
| Pathway | No. | Specific Risk | Likelihood | Consequence | Rating | Comments |
|---|---|---|---|---|---|---|
| P8 | 1 | Movement of live infected animals through processor for sea-ranching or seacage farming | 4 | 3 | 12 | |
| P8 | 2 | Transfer of virus to uninfected wild areas through inappropriate disposal of viscera, shells, mortalities and other processing wastes, including sale of by- products as bait to fisherman | 5 | 4 | 20 | Heat treated processed products and by-products are not an issue, but pre-processed by-products are an issue. Marine dumping at sea (not widely practised, but needs to be highlighted). EPA issues regarding proscribed wastes |
| P8 | 3 | Transfer of virus to uninfected wild areas through inappropriate disposal of process effluent water. | 3 | 3 | 9 | Inappropriate disposal - direct disposal untreated to the marine environment. Holding facility - outfall will become a high concentration point for the virus. |
| P8 | 4 | Virus transferred to wild by contaminated equipment | 3 | 3 | 9 | Contact time is important. Risk is lower. |
Pathway 9: Movement within and between processors.
| Pathway | No. | Specific Risk | Likelihood | Consequence | Rating | Comments |
|---|---|---|---|---|---|---|
| P9 | 1 | Movement of live infected animals (abalone and other species that may harbour the virus) between processors - local movements. | 5 | 3 | 15 | Likelihood and consequence depends on whether live/holding processor or cannery processor as bio- security and level of processing will be different. |
| P9 | 2 | Movement of live infected animals (abalone and other species that may harbour the virus) between processors - adjoining zones. | 5 | 3 | 15 | Likelihood and consequence depends on whether live/holding processor or cannery processor as bio- security and level of processing will be different. One live holding facility has two sites. |
| P9 | 3 | Movement of dead/ semi processed animals (abalone and other species that may harbour the virus) between processors - adjoining zones. | 4 | 2 | 8 | Happens quite often. Vic abalone processors take in both live and shucked abalone. Transferred to inter-state. One WA processor sends frozen meat overseas for canning. |
| P9 | 4 | Movement of dead/ semi processed animals (abalone and other species that may harbour the virus) between processors - interstate | 4 | 4 | 16 | |
| P9 | 5 | Movement of live infected animals (abalone and other species that may harbour the virus) between processors - interstate. | 5 | 5 | 25 | |
| P9 | 6 | Movement of dead/ semi- processed animals (abalone and other species that may harbour the virus) between processors - international. | 4 | 5 | 20 | |
| P9 | 7 | Movement of live infected animals (abalone and other species that may harbour the virus) between processors - international. | 5 | 5 | 25 | |
| P9 | 8 | Movement of contaminated equipment, vehicles and personnel introduces virus between processors | 5 | 3 | 15 | e.g. transport boxes/crates. Based on geographic zones Consequences=2 to 5. |
| P9 | 9 | Movement of contaminated transport water may carry virus particles between processors. | 5 | 4 | 20 |
Pathway 10: Other
| Pathway | No. | Specific Risk | Likelihood | Consequence | Rating | Comments |
|---|---|---|---|---|---|---|
| P10 | 1 | Inability to rapidly diagnose presence of virus | 0 | Not assessed at workshop | ||
| P10 | 2 | Live infected animals to wild overseas through export trade | 2 | 2 | 4 | Has to get from the overseas market to the wild. Farmers overseas may buy from the market for use on farms. |
| P10 | 3 | Live infected animals to overseas markets through export trade | 3 | 3 | 9 | Damage to reputation. Economic consequences |
| P10 | 4 | Failure to comply with protocols | 0 | Not assessed at workshop | ||
| P10 | 5 | Failure to implement recommendations of Codes of Practice | 5 | 4 | 20 | |
| P10 | 6 | Decision making risks not defined | 0 | Not assessed at workshop |
Notes:
1 nd=no data.
2 Illegal take of abalone is not considered further in this risk assessment as a Code of Practice is not a suitable mechanism to control such activities.
3 This document is available from the Library, DPI Queenscliff Centre, PO Box 114, Queencliff, Victoria 3225.
Acknowledgments:
This Information Note was developed by Gavine, F. Ingram, B. and Doroudi, M., May 2009.
