Protocol for the Translocation of Blue Mussels
February 2006
Fisheries Management Report Series No. 26
Authors: Neil Hickman & John Mercer
Preferred way to cite this publication:
Anon (2006) Victorian Protocol for the Translocation of Mussels. Fisheries Victoria Management Report Series No. 26.
ISSN 1448-1693
ISBN 1 74146 461 7
Introduction
Purpose
The purpose of this protocol is to mitigate the risks associated with the translocation of blue mussels for the purposes of aquaculture within and to the State of Victoria, Australia. A risk assessment has been used to guide the development of the protocol as part of the implementation of management plans for Victorian Aquaculture Fisheries Reserves (AFR). This document details the risk assessment process undertaken in relation to the translocation of mussels in Victoria, and the subsequent controls recommended for the translocation activities associated with the mussel aquaculture industry.
Scope
This protocol deals only with the translocation of mussels into and within Victoria, and only with those practices currently undertaken within the mussel farming industry. The types of translocation that occur in Victoria, and thus considered within the scope of this protocol, are detailed in Table 1.
In Victoria, mussel farming is a stated "beneficial use" under State Environmental Protection Policies (SEPPs). In the 1980s the State Government phased out the environmentally damaging practice of dredging blue mussels and encouraged the development of mussel aquaculture. Farming of the blue mussel (Mytilus galloprovincialis1) is taking place in all southern states including New South Wales, Victoria, Tasmania, South Australia and Western Australia. Mussels are a natural part of temperate Australian marine ecosystems. The environmental effects of mussel farming in Port Phillip Bay have been negligible after a period of 15 years (McKinnon et al. 2003). Therefore there is no major concern arising from the risks of mussels escaping, surviving or establishing feral populations. These are the most undesirable endpoints for non-native species (Hayes 1997; Anon 2003). The main risks considered are the cotransport of marine pests and diseases or deleterious genetic changes to existing populations.
All mussel translocations will be into government-approved AFRs that are licensed for mussel farming. Therefore, a decision has already been made that the risks to the ecosystem and biodiversity from the farming of blue mussels are acceptable and is, therefore, not part of this protocol. A summary of ecological benefits of mussel farminEvent may occur only inexceptional circumstancesg is presented.
Table 1. Potential mussel translocation activities in Victoria
| Translocation Activity | Translocation to | |
|---|---|---|
| 1 | Translocation of hatchery spat within Victoria | Licensed mussel growing areas in Victoria |
| 2 | Translocation of hatchery spat from interstate | As above |
| 3 | Translocation of wild mussels within Victoria | As above |
| 4 | Translocation of wild mussels from interstate | As above |
1 This species has been known by several names in Australiaincluding Mytilus edulis and M. planulatus. There is some question as to the valid name although recent geneticstudies indicate that the Australian form is closer to M. galloprovincialis. Some workers recognise the Australianform as a subspecies, M. galloprovincialis planulatus.
Definitions
For the purposes of this protocol, translocation is defined as "the deliberate human-assisted movement of a live aquatic organism using associated transport media." Other relevant definitions include:
AFR: Aquaculture Fisheries Reserve
Approved Authority/Laboratory: A laboratory in a Member Country that is approved by the Competent Authority to carry out diagnostic work on disease listed by the World Organisation for Animal Health (OIE).
Competent Authority: The National Veterinary Services, or other Authority of a Member Country, having the responsibility and competence for ensuring or supervising the implementation of the aquatic animal health measures recommended in the International Aquatic Animal Health Code (Anon 2003b).
Consequence: The outcomes of an event or situation expressed qualitatively or quantitatively, being a loss, injury, disadvantage or gain.
Disease: Clinical or non-clinical infection with one or more of the aetiological agents of the diseases listed in the Aquatic Animal Health Code, 2003 (OIE).
Endemic: Species restricted in distribution to a particular region.
Likelihood: Probability or frequency of a particular event or situation occurring.
Marine pest: Unwanted species that can be translocated with mussels (Listed in Hayes et al 2004).
Mussel hatchery spat: Small post-larval settled mussels below 2 mm in size that are usually sold to mussel farmers on 5 m long ropes.
Mussel juvenile: An intermediate sized mussel that is capable of being "fresh-water dipped" prior to translocation.
Mussel seed: Juvenile mussels of a size that farmers can still attach to growing ropes with"seeding socking".
Panmictic: A single genetic stock in which mating is random.
Quarantine: Maintaining a group of aquatic animals in isolation with no direct or indirect contact with other aquatic animals, in order to undergo observation for a specified length of time and, if appropriate, testing and treatment, including proper treatment of the effluent waters.
Risk: The chance of undesirable events, expressed as a function of the likelihood and consequence of such events.
Risk Assessment: Procedure where estimates of likelihood and consequence of an event are combined to arrive at a given level of risk. Control methods are then developed to rank and reduce the risks.
TEP: Translocation Evaluation Panel. An expert evaluation panel who will: evaluate all risk assessments; assess all translocation protocols; determine if proposed translocations comply with established protocols; and consider all other translocation proposals on their merits.
VSQAP: Victorian Shellfish Quality Assurance Program
Need
Legislation
Key Victorian legislation surrounding the translocation of mussels includes:
- The Fisheries Act 1995, which regulates the commercial mussel industry, including the stocking of mussels in Crown waters, and establishes a number of objectives aimed at achieving ecologically sustainable development with reference to the maintenance of aquatic processes and genetic diversity; and
- The Flora and Fauna Guarantee Act 1988, which provides for the protection and conservation of native flora and fauna.
All translocation activities described are required to be licensed under the Fisheries Act 1995 and are subject to the Fisheries Regulations 1998. Licences are subject to any conditions as specified by the Secretary, of the Victorian Department of Environment and Primary Industries (DEPI). The Fisheries Division, DEPI, has a lead role in developing a protocol for the translocation of mussels in Victoria.
Policy
The Victorian Aquaculture Strategy (1998) highlighted the need to "establish a risk management approach to conserve biodiversity" and further established a goal ʺto ensure ecologically sustainable development". The Victorian area based Management Plans for all AFRs will provide the policy framework and support for the management of the Victorian mussel industry. It has been recommended by Fisheries Victoria and Industry that a Translocation Protocol be developed for the mussel aquaculture industry.
The Guidelines for Assessing Translocations of Live Aquatic Organisms in Victoria (the Guidelines) (Anon 2003) provides the policy framework for developing a protocol for the translocation of mussels into and within Victoria. The protocol aims to facilitate repeated translocation events within licensed aquaculture and commercial fishery activities.
All translocation protocols are being developed to fulfil the requirements of the Guidelines.
The Australian Blue Mussel and the Aquaculture Industry
The blue mussel occurs in temperate Australia (all states except Queensland and the Northern Territory). Evidence presently available supports the taxonomic identity of Australian (and New Zealand) populations of blue mussels as Mytilus galloprovincialis. This is based on two decades of research using mutivariate analysis, enzymes and genes (Mc Donald et al. 1991; Seed 1992; and Gardner 1992, 2004). The Mytilus genus has been described as a remarkable species complex that has circumpolar distribution in the Northern and Southern Hemispheres (Wilson 1998).
The blue mussel (M. galloprovincialis, previously known as Mytilus planulatus Lamark 1818, and Mytilus edulis planulatus Soot-Ryan 1955) has been found in middens which pre-date European settlement of Australia and New Zealand. Records exist for New South Wales and Victoria (Donner and Junger 1981); South Australia (Hope et al. 1977); Tasmania (Colhoun et al 1982); and New Zealand (Gardner 2004). This mussel often forms dense beds that are important components of many coastal marine ecosystems. Mytilus galloprovincialis is endemic to Australia and panmictic in breeding.
Many mussel aquaculture industries throughout the world farm the Mytilus genus. In Victoria the mussel aquaculture industry was first developed in Port Phillip Bay in the early 1980s, and in the future could be undertaken in eight specific AFRs, the locations of which are shown in Figure 1. In the past, industry has been entirely reliant on annual catches of mussel spat caught naturally in Port Phillip Bay. In recent years some poor spat-falls have resulted in small-scale production of mussel spat in one Tasmanian and two Victorian hatcheries. In all cases, both wild and hatchery mussel spat are grown on surface-floated long-lines in Victorian AFRs.
The current mussel industry utilises 938 ha of water to produce approximately 1,400 tonnes of mussels annually. The Victorian mussel industry is currently the largest producer of mussels in Australia, but significant development is also occurring in Tasmania and South Australia. The Environment Conservation Council (Victoria) Marine Coastal and Estuarine Investigation: Final Report (Anon 2000) has recommended 12 sites for aquaculture development. The total area recommended for aquaculture is 2,682 ha. It is therefore anticipated that the Victorian mussel industry will expand significantly in the near future. All mussel farmers hold an Aquaculture (Crown Land - Bivalve Shellfish) Licence.
In the early years of the Victorian mussel aquaculture industry, all AFRs for farming mussels were located in Port Phillip Bay where the juvenile mussels were caught by hanging ropes in the water from late winter to early spring to catch mussel larvae. In 1993 the Flinders Aquaculture Fisheries Reserve (FAFR) was opened in Westernport which is located 60 km to the east of Port Phillip Bay (Figure 1). Seed mussels are translocated annually from spat collection sites in Port Phillip Bay to be on-grown at the FAFR. This was the first time that industry translocated mussels for farming outside of Port Phillip Bay.
This industry practice of translocating mussels raised concerns that this could be a vector for translocating marine pests, known to occur in Port Phillip Bay, to other Victorian bays. A consortium of Victorian Mussel Farmers supported a project that was funded by the National Heritage Trust and the Victorian Department of Natural Resources and Environment. Robust toxicological methodology was used to assess a range of treatments designed to kill exotic pests of concern but not harm the juvenile mussels. This resulted in a protocol for killing marine pests on mussel ropes (Gunthorpe et al. 2001) so that the translocation of mussels from Port Phillip Bay to Westernport could continue.
In 2001 and 2002, small quantities of mussel spat were produced in a Tasmanian hatchery for on-growing in Victorian AFRs. In 2003 and 2004, Victorian hatcheries also produced trial quantities of mussels for on-growing in Victorian AFRs. The demand for hatchery-produced spat is likely to increase in coming years particularly if there is any shortage of wild-caught spat.
The mussel aquaculture industry makes a significant contribution to regional economies, particularly on the Bellarine Peninsula. Production is currently very dependent on natural spat-fall, and approximately $4M of cultured mussels is produced annually. Currently most mussel production is for domestic consumption, but recently all Victorian farming areas been given export accreditation by the Australian Quarantine and Inspection Service (AQIS). As production increases it is the intention of the Victorian industry to develop new markets including export markets.
In Victoria, approximately 30 mussel farming licences have been issued, and the industry directly employs 53 full-time, and up to 51 part-time personnel across Victoria. In post-harvest, 19 permanent and 18 casual staff are employed. It is predicted that intensive aquaculture of mussels using new growing areas and new growing technology is likely to see a three-fold expansion of the industry over the coming decade.
With the projected increase in the size of the Victorian mussel industry, it is likely that requests to translocate hatchery mussel spat and "wild" mussel seed from interstate and within Victoria will increase in coming years. With the possibility of farmers holding leases in existing and new areas, it is also likely that farmers will wish to move juvenile mussels in order to optimise production (as is practiced in many shellfish aquaculture industries).
General Risks Associated with Translocation in Victoria
In general, the risks associated with the translocation of aquatic organisms have been stated as:
- Establishment of feral populations;
- Environmental impacts from release of translocated species;
- Translocation of associated species (the most potentially harmful being marine pests that have been ranked highly as national priority pests (Hayes et al. 2004));
- Disease and parasite introduction;
- Genetic shift in wild populations;
- Chemical release; and
- Socio-economic impacts from release of translocated species.
The translocation of an aquatic organism at its broadest definition encompasses any human assisted movement of that organism. The translocation of aquatic organisms is recognised as a potentially threatening process to the environment, particularly where such translocations occur outside the natural range of the species being translocated. As aquaculture and fisheries enhancement present opportunities to utilise a range of species within and outside their natural range, in a variety of farming systems, it is important to identify and manage the risks associated with this activity. It is also suggested that the aquaculture and fishing industry sectors take an active role in managing this issue to ensure community confidence is maintained.
Specific Risks Associated with Mussel Translocation in Victoria
Given the widespread distribution of blue mussels
(M. galloprovincialis) in southern Australia, including aquaculture production in all southern states, the risks that mainly apply to culturing a species outside its natural range will not apply. Similarly the risks of escape or establishing a feral population will not apply where a species is a common component of coastal ecosystems.
In the case of mussel translocations into approved AFRs, on the basis of a literature review, it was determined that important risks that would need to be considered were spread of disease, toxic algae and exotic pests, and changes to genetic identity. Risks to the environment from mussel aquaculture were not assessed in this protocol because they were considered in a separate process which established both AFRs and Marine Parks (Anon 2000).
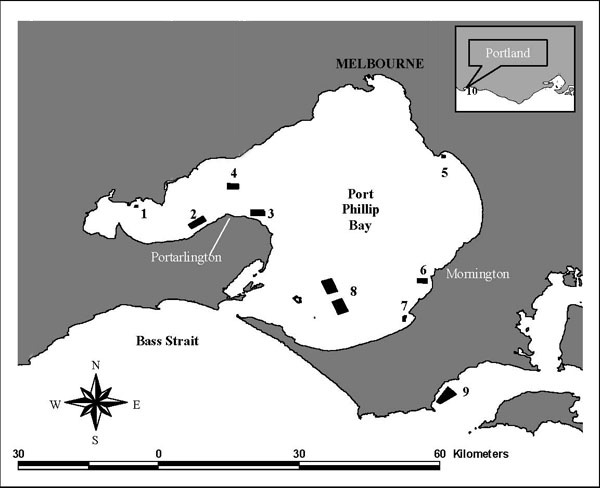
Figure 1. Location of the existing AFRs of Clifton Springs (2), Grassy Point (3), Kirk Point Werribee (4); Beaumaris (5), Dromana (7), and Flinders (9); plus the proposed AFRs of Bates Point (1), Mount Martha(6), Pinnace Channel (8), and Portland (10).
Summary of Risk Assessment
Methodology
A risk assessment was conducted according to Departmental Risk Management Strategic Framework and Process, which is based on the Australian/New Zealand Standard for Risk Management (AS/NZS 4360:1995). An expert panel was convened to cover knowledge of mussel translocations and the main areas of perceived risk. The panel included:
Dr Mehdi Doroudi. Director, Marine and Freshwater Systems, PIRVic (Formally Biosecurity Victoria) (Disease).
Dr Michael Goddard. Senior Scientist, PIRVic, Attwood Centre. (Genetics)
Dr Michaela Dommisse. Senior Scientist, Department of Sustainability and Environment. (Marine pests / ecology)
Mr Michael Callan. Mussel farmer. (Mussel aquaculture industry).
Mr Anthony Forster. Aquaculture Manager, Fisheries Victoria. (Fisheries policy).
Mr John Mercer. Marine Extension Officer, Fisheries Victoria. (Shellfish hatcheries / Victorian mussel treatment protocol).
Mr Neil Hickman. Senior Science Officer. Primary Industries Research Victoria. (Mussel aquaculture research and risk management facilitation).
The evaluation of specific risks associated with the translocation of mussels was undertaken using standard risk assessment criteria. Experts ranked risks on their likelihood and consequence, which was then summed to give a risk rating as per the tables below.Likelihood Rating
| Likelihood Rating | Description | Likelihood of Occurrence |
|---|---|---|
| 1 | Rare | Event may occur only in exceptional circumstances |
| 2 | Unlikely | The event may occur atsome time, say once in 10 years |
| 3 | Moderate | The event should occur at some time, say once in three years |
| 4 | Likely | The event will probably occur in most circumstances, say once a year |
| 5 | Almost Certain | The event is expected to occur in most circumstances, say many times a month |
Consequence Rating
| Consequence Rating | Description | Environmental |
|---|---|---|
| 5 | Catastrophic | Serious long]term or widespread environmental harm. |
| 4 | Major | Significant environmental harm with long]termrecovery |
| 3 | Moderate | Moderate harm with midtermrecovery |
| 2 | Minor | Transient environmental harm |
| 1 | Insignificant | Brief pollution with effective remediation |
Risk Ratings
| Risk Ranking | Score | Assessment |
|---|---|---|
| High | >8 | Requires detailed research,planning and decision making atsenior levels of management |
| Significant; | 7 | Senior management attention and action needed |
| Moderate | 6 | Management responsibility maybe specified |
| Low | <5 | No major concern |
The results of the risk assessment are detailed in Appendix 1. All identified high risks were in the categories of disease and marine pests.
Mussel Disease
There are international and national protocols concerning identification and notification for molluscan diseases (Anon 2003b, c). To date, no notifiable diseases have been identified in Australian mussels.
Marine Pests
The translocation of marine pests has been recognised as a major threat to marine ecosystems throughout the world. In Australia this threat is being addressed by implementing the Intergovernmental Agreement on a National System for the Prevention and Management of Marine Pest Incursions (IGA) signed in Darwin on 15 April 2005. The potential for marine pests to entrain in water, hard surfaces (ropes, mussels) and sediment associated with mussel stock and gear was used to assess the risk of their translocation. Marine pests were considered in different functional groups that reflect their ability to be transported by a particular vector (Appendix 2).
Identified High Risks for Mussel Translocations
The two high risks identified in the risk assessment for translocations within Victoria were:
Risk 1. Translocation of wild mussel seed could transmit disease; and
Risk 2. Translocation of wild mussel seed could transmit marine pests.
The four high risks for translocations from interstate were:
Risk 3. Translocation of hatchery mussel spat could transmit disease.
Risk 4. Translocation
of wild mussel seed could transmit disease.
Risk 5. Translocation of hatchery mussel spat could transmit marine pests.
Risk 6. Translocation of wild mussel seed could transmit marine pests.
Controls for High Mussel Translocation Risks
Control measures for these risks are detailed in Appendix 1, and can be summarised as follows:
Controls for Mussel Translocations within Victoria
Risk 1: Translocation of wild mussels could transmit disease.
Suggested control for Risk 1:
- Biosecurity Victoria is not currently concerned with translocation of wild mussels within Victoria. In the event of an outbreak of a notifiable disease in mussels in this state, Biosecurity Victoria will require the same testing regime that is applied to interstate movement of hatchery mussel spat and wild mussels (See controls for Risks 3 and 4 below).
Risk 2: Translocation of wild mussels could transmit marine pests.
Suggested controls for Risk 2:
- Apply the documented protocol (Gunthorpe et al. 2001) of 2 hours freshwater immersion of seed mussels followed by 12 hours in air.
- Only allow mussel translocation if VSQAP algal monitoring shows toxic algae below alert levels (Appendix 3)
Controls for Mussel Translocations from Interstate
Risk 3: Translocation of hatchery mussel spat could transmit disease.
Suggested control for Risk 3:
- Implement hatchery disease testing protocol: All unexplained mussel mortality within the hatchery must be declared by the supplier. One hundred and fifty spat will be tested histopathologically by a competent veterinary authority for the detection of infectious agents (Appendix 4). Translocation permitted if there is no unexplained mortality and no diseases are detected.
Risk 4: Translocation of wild mussels could transmit disease.
Suggested control for Risk 4:
- Implement mussel testing protocol for disease: One hundred and fifty seed mussels will be tested histopathologically by a competent veterinary authority for the detection of infectious agents (Appendix 4). Translocation will be permitted if free of disease.
Risk 5: Translocation of hatchery mussel spat could transmit marine pests.
Possible controls for Risk 5:
- To mitigate the possible contamination with Pacific oysters, the hatchery will need protocols and documentation to show separation of batches and equipment. A completed Hatchery Compliance Report (Appendix 5) will be required.
- Only allow mussel translocation if toxic algae are below alert levels (Appendix 3).
Risk 6: Translocation of wild mussels could transmit marine pests.
Possible controls for Risk 6:
- Conduct a full risk assessment to meet the requirements of the Victorian Translocation Evaluation Panel (Appendix 6). Prohibit translocating wild seed until risk treatment plans can demonstrate that the risks posed by pests can be addressed. It must be demonstrated either that protocols to exclude the pests of concern have been developed, or an acceptable survey method shows that the pests of concern do not exist in the area where the seed mussels are growing.
- Toxic algae can be controlled by sourcing seed from areas covered by the relevant stateʹs routine Shellfish Quality Assurance Program (SQAP) algal monitoring or testing for toxic algae of concern. Only allow translocations if levels of toxic algae are below trigger levels (Appendix 3).
- Apply standard freshwater dip and air-drying procedures to mussels sources from interstate (Gunthorpe et al 2001).
Protocols for Mussel Translocation in Victoria
In the risk assessment, high risks associated with translocating mussels within and into Victoria were identified and prioritised. Possible control measures were also identified. Some of the suggested control measures have been fully developed, documented and implemented. Other controls can be readily put in place. These form the basis of the recommended translocation protocols for mussels as described below.
Some mussel translocations from interstate will require additional work in order to finalise a translocation protocol.
Translocation of Mussels within Victoria
Hatchery Mussel Spat within Victoria
Translocation of Victorian hatchery mussel spat will be permitted provided the following conditions are met:
- Only the native blue mussel M. galloprovincialis is used as broodstock.
- Mussel spat intended for producing mussels for human consumption is sold only to licensed mussel farmers and is grown in designated AFRs.
- All water used in hatcheries must be pre-filtered to 50 µm.
- Aquaculture licence holders will be required to report on the presence of exotic/ noxious pests as a licence condition.
Wild Mussels within Victoria
Translocation of Victorian wild-caught mussels will be permitted provided the following conditions are met:
- There has been no reported mussel mortality in the area where the mussels are being grown.
- In the event of reported mortality of mussels, testing by a competent veterinary authority rules out disease as the cause.
- Moving cultured mussels within Port Phillip Bay is permitted without any restrictions.
- The documented protocol (Gunthorpe et al. 2001) for the treatment of mussel ropes and mussels is applied when moving mussels between bays. (NB: This involves 2 hours of freshwater immersion of seed mussels followed by 12 hours in air, prior to transplanting).
- Mussel translocation allowed only if algal monitoring shows toxic algae below alert levels in the area where the mussels are being held (Appendix 3). (NB: VSQAP monitoring will cover growing areas.)
Translocation of Mussels into Victoria
Hatchery Mussel Spat from Interstate
Translocation of mussel spat from an interstate hatchery will be permitted provided that the following conditions are met:
- Only the native blue mussel M. galloprovincialis is used as broodstock.
- Mussel spat intended for producing mussels for human consumption is sold only to licensed mussel farmers and is grown in designated AFRs.
- All water used in hatcheries must be pre-filtered to 50 µm.
- A declaration by the supplier of all unexplained mussel mortality is supplied; and
- A mussel disease testing protocol isimplemented for each batch of mussels andincludes the following:
- One hundred and fifty spat are tested histopathologically by a competent veterinary authority and a certificate of disease testing is provided to Biosecurity Victoria and Fisheries Victoria. There is no unexplained mortality and testing does not identify diseases of concern.
- A hatchery disease testing protocol is implemented and includes the following:
- A hatchery compliance report (Appendix 5) is forwarded to Fisheries Victoria and demonstrates the following:
- There has been a complete separation of all production facilities to separate mussels from other species. (Particularly important in a multi-purpose hatchery that also produces Pacific oysters); and
- Toxic algae are below alert levels (Appendix 3).
Wild Mussels from Interstate
Translocation of wild mussels from interstate will be permitted provided that all of the following conditions are met:
- A mussel disease testing protocol is implemented for each batch of mussels and includes the following:
One hundred and fifty spat are tested histopathologically by a competent veterinary authority and a certificate of disease testing is provided to Biosecurity Victoria and Fisheries Victoria. Mussels must be free of disease to allow translocation. - A full risk assessment (Appendix 6) is conducted by the proponent wishing to translocate mussels in order to demonstrate that any threat posed by marine pests that can not be controlled by the Victorian Freshwater and Air Treatment Protocol can be mitigated. The risk assessment and proposed mitigation methods must be approved by the Victorian TEP.
- Monitoring shows that toxic algae are below alert levels (Appendix 3) in the source area.
- The Victorian Freshwater and Air Treatment Protocol is applied to all seed prior to translocation.
Further Information and Issues
Fish Health Certification Process for Hatchery Produced Mussels
- The aquaculture licence holder endorsed for mussels must obtain a fish health accreditation report prior to bringing hatchery-produced mussels into Victoria for aquaculture purposes. This report shall be prepared by a veterinarian approved by Fisheries Victoria following a visit to the hatchery (Appendix 5). Each proposed consignment will be assessed for the presence of clinically abnormal shellfish and include a report describing the history of mortality, an explanation of previous mortality and reference to those samples previously preserved. If in the opinion of the veterinarian the fish health status is unsatisfactory, the consignment will not be approved for translocation to Victoria.
- Certification of disease testing of the consignment of mussels to be translocated is to be provided to Fisheries Victoria from the relevant veterinarian.
- The cost of preparing the fish health accreditation report will be borne by the Aquaculture Licence holder endorsed for mussels. A copy of the report shall be forwarded to Victorian Fisheries, Aquaculture Section, prior to mussels being brought into Victoria.
- NB: In the event of an outbreak of a notifiable disease in mussels in a Victorian hatchery, Biosecurity Victoria will prohibit the translocation of hatchery produced mussel spat.
| Currently the only approvals that have been given for importation of mussels from interstate have been for the hatchery-produced mussel spat from Tasmania. |
Animal Welfare
Animal welfare issues do not apply to mussels. However, mussels are supplied live to consumers and temperature control is maintained through the supply chain in order to maintain seafood safety standards.
Victorian Shellfish Quality Assurance Program (VSQAP)
This program ensures that mussels grown in AFRs are fit for human consumption. The risks from pathogens and toxic algae are controlled by regular monitoring and farm closures when adverse conditions prevail. This program provides background information on all species of toxic algae at commercial mussel farms on a fortnightly basis; not just the species which have been identified as high risk exotic marine pests. Therefore the risks of translocating any toxic algae from any AFR are mitigated by regular routine monitoring of all the potentially threatening species. No mussel translocations would be permitted if toxic algae exceeded the VSQAP "alert" levels. If blooms of toxic algae have not occurred in recent times, there is little likelihood of toxic algal cysts being present in the water column at farm sites.
Ecological Benefits of Mussel Farming
The Victorian Government phased out mussel dredging because it was considered to be environmentally damaging, and encouraged the development of mussel farming. The environmental effects of blue mussel aquaculture in Port Phillip Bay have been found to be negligible (McKinnon et al. 2003). Furthermore, in Port Phillip Bay, extensive shellfish beds (including mussels) have declined significantly in recent years. Therefore, mid-water mussel aquaculture offers the opportunity to replenish benthic mussel beds by acting as a source of broodstock mussels to provide large numbers of planktonic mussel larvae that could re-establish the lost benthic mussel beds.
In addition, the reduction of nitrogen in Port Phillip Bay is a Victorian Government priority (Harris et al. 1996). Mussel farming has the potential to remove approximately 12 tonnes of nitrogen for every 1,000 tonnes of wet weight of mussels harvested. Therefore, on balance, mussel farming provides more ecological benefits than ecological threats.
Translocation of Mussels for Hatchery Research Purposes
The issue of future possible translocation of broodstock mussels to Victorian land-based hatcheries for genetic research purposes was raised during risk assessment workshops. None of these mussels would be released to the marine environment, but in future their offspring could be released for aquaculture purposes. This was outside the scope of the current risk assessment that was specifically directed at assessing the translocation of mussels into and within Victoria, and only addressed the practices currently undertaken within the mussel farming industry. In relation to future hatchery research using translocated mussels, the following three recommendations are made:
- Any translocation of mussels for research purposes should also follow the protocols explained in this report.
- A hatchery conducting genetic research on mussels must be able to hold these mussels under adequate quarantine conditions.
- Future genetic research on hatchery mussels and the potential outcomes would require a review of genetic risks, particularly if the intent was to grow selectively bred or sterile progeny on mussel farms. Current and proposed translocation practices considered in this report do not constitute a high genetic risk for mussels.
Acknowledgements
This report was written by N J Hickman, with extensive use of material prepared by J A Mercer. The author wishes to thank the other members of the expert risk assessment panel: Dr Mehdi Doroudi (Director, Marine and Freshwater Resources Platform of Primary Industries Research Victoria); Dr Michael Goddard (PIRVic, Attwood); Dr Michaela Dommisse (Department of Sustainability and Environment); Mr Michael Callan (Director, Sitelair Pty Ltd); Mr Anthony Forster (Aquaculture Manager, Fisheries Victoria) and Mr John Mercer (Marine Extension Officer, Fisheries Victoria). The author also acknowledges the advice and assistance provided by Mr Lachlan McKinnon who prepared the Victorian Eel Translocation Protocol.
References
Anon (2000) Marine coastal and estuarine investigation: final report. Environment Conservation Council (Victoria).
Anon (2003) Guidelines for assessing translocations of live aquatic organisms in Victoria. The State of Victoria, Department of Environment and Primary Industries 2003.
Anon (2003b) International Aquatic Animal Health Code, Office International des Epizooties, Paris.
Anon (2003c) Diagnostic Manual for Aquatic Animal Diseases, Office International des Epizooties, Paris.
Anon (2003d).Victoriaʹs Arrangements for the Management of Aquatic Animal Disease Emergencies. The State of Victoria, Department of Environment and Primary Industries 2003.
Anon (in press). Marine Pest Monitoring Manual. Australian Government Department of Agriculture, Fisheries and Forestry and New Zealand Ministry of Agriculture and Forestry.
Australian/New Zealand Standard for Risk Management (AS/NZS 4360:1995). Standards Australia/Standards New Zealand
Colhoun EA, Turner E, and Van de Geer (1982) Late Pleistocene marine molluscan faunas from sites in Tasmania. Pap. Proc. R. Soc. Tasm. 116: 91 - 96.
Department of Environment and Primary Industries (2004). Marine Biotoxin Management Plan 2004. Fisheries Victoria Management Report Series No. 11.
Gardner JPA (1992) Mytilus galloprovincialis (Lmk) (Bivalvia, mollusca): The taxonomic status of the Mediterranean mussel. Ophelia 35: 219-243.
Gardner JPA (2004) A historical perspective of the genus Mytilus (Bivalvia, Mollusca) in New Zealand: Multivariate morphometric analysis of fossil midden and contemporary blue mussels. Biological Journal of the Linnean Society 82: 329-334.
Gunthorpe L, Mercer J, Rees C, and Threodoropolous T (2001) Best practices for the sterilisation of aquaculture farming equipment: A case study for mussel ropes. Marine and Freshwater Resources Institute Report No 41. Marine and Freshwater Resources Institute: Queenscliff.
Donner J and Junger H (1981) Radiocarbon dating of marine shells from south-eastern Australia as a means of dating relative sea-level changes. Ann. Acad. Sci. Fenn. (Geol. Geogr) 131: 1-44.
Harris G, Batley G, Fox D, Hall D, Jernakoff P, Molloy R, Murray A, Newell B, Parslow J, Skyring G and Walker S (1996) Port Phillip Bay Environmental Study Final Report. CSIRO, Canberra, Australia.
Hayes KR (1997) A review of the Ecological Risk Assessment Methodologies. Centre for Research on Introduced Marine pests, Technical Report No
13. CSIRO Division of Marine Research, Hobart.
Hayes KR, Sliwa C, Migas S, McEnnulty F and Dunstan P (2004) National priority pests: Part II Ranking of Australian marine pests. CSIRO Marine Research 2004. Published by Australian Government Department of Environment and Heritage, John Gorton Building, King Edward Terrace, Parkes ACT 2000, Australia.
Hope JR, Lampert RJ, Edmonson E, Smith MJ and Van Tets (1977) Late Pleistocene faunal remains from Seton rock shelter, Kangaroo Island, South Australia. J. Biogeogr. 4: 363-385.
McDonald J H, Seed R, Koehn R K (1991) Alloenzyme and morphometric characters of three species of Mytilus in the Northern and Southern Hemispheres. Marine Biology 111: 323-333.
McKinnon LJ, Parry GD, Leporati SC, Heislers S, Werner GF, Gason ASH, Fabris G and OʹMahony N (2003) The environmental effects of the blue mussel (Mytilus edulis) aquaculture in Port Phillip Bay. Fisheries Victoria, Research Report series No.
1. Seed R (1992) Systematics, evolution and distribution of mussels belonging to the genus Mytilus: an overview. American Malacological Bulletin 9, 123-137.
Victorian Aquaculture Strategy (1998). Fisheries Victoria. Department of Natural Resources and Environment.
Wilson BR (1998) Order Mytiloida. In ʹMollusca: the Southern Synthesis.ʹ Fauna of Australia Vol. 5 (Ed Beesley PL) pp 250-253. CSIRO Publishing 1998.
Appendix 1. Risk Assessment Study ofMussel Translocation in Victoria
Application of the Guidelines for Assessing Translocations of Live Aquatic Organisms in Victoria (the Guidelines) (Anon 2003).
In line with the Guidelines, a risk assessment has been conducted to identify the major risks that will need to be addressed in order to develop Mussel Translocation Protocols for Victoria. Six possible translocation scenarios were assessed. The risks associated with mussel seed were deemed to be the same as the risks for juvenile or adult mussels, so the latter were combined to leave four main types of translocation for consideration. During the risk assessment, each of these four translocations was repeatedly considered against each identified risk.
The main objectives in developing a mussel translocation protocol will be to preserve the natural and farmed stocks of Victorian mussels, and to ensure that any mussel translocations do not adversely impact the Victorian marine environment. This will be done by evaluating the major translocation risks posed by introducing diseases, marine pests or other undesirable changes to the mussel stocks themselves (such as major genetic shift). Brief consideration was given to environmental/ecological and social/economic threats from mussels, but these risks were deemed not to be applicable (See end of following table).
Table 2. The four translocation scenarios used in this risk assessment
| Translocation Activity | Translocation to | |
|---|---|---|
| 1 | Translocation of hatchery spat within Victoria | Licensed mussel growing areas in Victoria |
| 2 | Translocation of hatchery spat from interstate | As above |
| 3 | Translocation of wild mussels within Victoria | As above |
| 4 | Translocation of wild mussels from interstate | As above |
Major Risk Category - Impact of Mussel Translocations by Industry Spreading Diseases
NB: Like. = likelihood and Conseq. = consequence.
| Risk Category | Specific Risk | Like. | Conseq. | Risk Rating | Comments and Possible Controls |
|---|---|---|---|---|---|
| Disease | Translocation of hatchery mussel spat within Victoria can spread disease. | 1 | 4 | 5 | There are no cases of mussel mortality that can be attributed to disease to date in Victoria. To date, no notifiable diseases have been found in Australian mussels. All known notifiable diseases (including molluscs) have been listed in Victorian Fisheries Regulations 1998 (Appendix 4). Possible mussel disease agents include Martelia sydneyi and Perkinsus olseni; however these have not been reported in mussels in Australia. With the exception of Perkinsus, most Australian notifiable molluscan diseases are believed to be highly species specific. The only known case of shellfish mortality caused by a notifiable disease in Victoria was the infection of flat oysters (Ostrea angasi) with Bonamia sp. Possible controls: |
| Disease | Translocation of hatchery mussel spat from interstate can spread disease. | 2 | 4 | 6 | There are no cases of mussel mortality that can be attributed to disease to date in Victoria. To date, no notifiable diseases have been found in Australian mussels. All known notifiable diseases (including molluscs) have been listed in Victorian Fisheries Regulations (Appendix 4). Possible mussel disease agents include Martelia sydneyi and Perkinsus olseni; however these have not been reported in mussels in Australia. With the exception of Perkinsus, most Australian notifiable molluscan diseases are believed to be highly species specific. Recently, outbreaks of notifiable molluscan diseases have occurred in other states, including Perkinsus olseni in abalone, Martelia sydneyi in Sydney rock oysters and Microcytos roughleyi (winter mortality disease) in Sydney rock oysters. The international literature shows that some species of Marteilia can infect mussels. Possible controls: |
| Disease | Translocation of wild mussels within Victoria can spread disease. | 4 | 4 | 8 | It is accepted that this will be a high risk in the event of an outbreak of any diseases specified in Appendix 4. Possible controls: |
| Disease | Translocation of wild mussels from interstate can spread disease. | 4 | 4 | 8 | Recently, outbreaks of notifiable molluscan diseases have occurred in other states, including Perkinsus olseni in abalone, Martelia sydneyi in Sydney rock oysters and Microcytos roughleyi (winter mortality disease) in Sydney rock oysters. The international literature shows that some species of Marteilia can infect mussels. Possible controls: |
Major Risk Category - Impact of Mussel Translocations by Industry on Accidental Transport of Marine Pests
|
Risk Category | Specific Risk | Like. | Conseq. |
Risk Rating | Comments and Possible Controls |
|---|---|---|---|---|---|
|
Marine Pests |
Translocation of hatchery spat within Victoria can spread marine pests. (With reference to three types of vector, namely: water; gear/equipment; and sediment). | 1 | 4 | 5 | No water or sediment vectors involved. Rope and mussels are the possible vectors. Ropes are cleaned and dried (weeks) prior to use. Representative unwanted species for functional groups are shown in Appendix 2. The main pests of concern in Victoria are the North Pacific seastar (Asterias amurensis), the Mediterranean fan worm (Sabella spallanzanii) and the brown seaweed (Undaria pinnatifida). These are all capable of fouling hard surfaces.There is also a risk of moving extremely small volumes of exotic toxic algae in mussel "shell liquor." Victorian hatcheries are not permitted to culture Pacific oysters. Suggested controls: 2) If fortnightly VSQAP monitoring for listed toxic species (which includes some exotic species listed as pests) shows toxic algae above "trigger levels" (Appendix 3) at local farm sites, the hatchery water should be examined for toxic algae. The translocation will be permitted if the algal count is below the required VSQAP "trigger level." |
|
Marine Pests | Translocation of hatchery seed from interstate can spread marine pests. (With reference to three types of vector, namely: water; gear/equipment; and sediment). | 3 | 4 | 7 | As above, with more emphasis on Pacific oysters if the mussel spat comes from a multipurpose hatchery that also produces Pacific oysters. Suggested controls: 2) In addition, the hatchery will need protocols and documentation to show separation of batches and equipment. A completed Hatchery Compliance Report (Appendix 5) will be required. |
|
Marine Pests |
Translocation of wild seed within Victoria can spread marine pests (With reference to three types of vector, namely: water; gear/equipment and sediment). | 5 | 4 | 9 | Translocation of mussels from Port Phillip Bay to Westernport was identified as high risk for translocating marine pests. This issue was the subject of National Heritage Trust funded research (MAFRI Report 41) which developed treatment protocols. Graded seed is free of sediment, but seeded ropes will have some associated sediment. The freshwater washing of seeded ropes will remove some sediment, but the main factor in preventing the translocation of undesirable toxic algal cysts would be the VSQAP monitoring program. The main vectors are the mussel shell surfaces and water present as internal "shell liquor." Suggested controls: 2) Only allow mussel translocation if VSQAP algal monitoring shows toxic algae below alert levels (Appendix 3). |
|
Marine Pests | Translocation of wild seed from interstate can spread marine pests (With reference to three types of vector, namely: water; gear/equipment; and sediment). | 5 | 4 | 9 | The Victorian Freshwater and Air Treatment Protocol will not kill exotic shellfish such as Pacific oysters, greenlip mussels or the New Zealand screw-shell. The incidence of toxic algal blooms is greater in some states (e.g. South Australia and Tasmania). Graded seed is free of sediment, but seeded ropes will have some associated sediment. The freshwater washing of seeded ropes will remove some sediment, but the main factor in preventing the translocation of undesirable toxic algal cysts would be the state conducting a SQAP that includes routine monitoring for toxic algae. The main vectors are the mussel shell surfaces and water present as internal "shell liquor". Possible controls: 2) Toxic algae can be controlled by sourcing seed from areas covered by Victoria's routine SQAP algal monitoring, or testing the water in the source area for toxic algae of concern. Only allow translocations if levels of toxic algae are below trigger levels (Appendix 3). 3) Apply the Victorian Freshwater and Air Treatment Protocol to interstate mussels. |
Major Risk Category - Impact of Mussel Translocations by Industry on Genetic Integrity of Mussel Populations
|
Risk Category | Specific Risk | Like. | Conseq. |
Risk Rating | Comments and Possible Controls |
|---|---|---|---|---|---|
| Genetics | Translocation of hatchery seed within Victoria can adversely affect genetic integrity of mussel populations. The following three questions should | 1 | 3 | 4 | Two decades of research using multi-variate analysis, enzymes and genes supports the taxonomic identity of Australian and New Zealand populations of blue mussels as Mytilus galloprovincialis. The blue mussel has been found in middens which pre-date European settlement of Australia and New Zealand.
Records exist for New South Wales and Victoria (Donner and Junger 1981); South Australia (Hope et al 1977); Tasmania (Colhoun et al 1982); and New Zealand (Gardner 2004). It often forms dense beds that are important components of many coastal marine ecosystems. Possible controls: |
| Genetics | Translocation of hatchery seed from interstate can adversely impact genetic integrity of mussel populations. | 2 | 3 | 5 | Although of minor consequence, distance is likely to increase any potential genetic risk. This should not be a major issue. |
| Genetics | Translocation of wild seed within Victoria can adversely impact genetic integrity of mussel populations. | Possible controls: | |||
| Genetics | Translocation of wild seed from interstate can adversely impact genetic integrity of mussel populations. | As above |
Other Possible Risks Posed by Industry Translocation of Mussels
|
Risk Category | Specific Risk | Like. | Conseq. |
Risk Rating | Comments and possible controls |
|---|---|---|---|---|---|
|
Threaten Social / Economic Assets | Any translocations | NA | NA | NA | Any damage caused to the mussel industry by translocating disease or pests could cause loss of jobs(social impact), and loss of revenue (economic impact). However these would be secondary risks to the primary risks of disease, marine pests or genetic damage. The translocation of mussels into environments where they have existed for thousands of years was not seen as a threatening process that could have major negative social or economic consequences. |
|
Chemical residue | Any translocations. | NA | NA | NA | No chemical residues used in aquaculture could be identified for hatchery mussel spat or seed mussels collected from the environment. |
|
Animal welfare | Any translocations | NA | NA | NA | Does not apply to bivalve shellfish |
| Research | Translocation of mature adult mussels to a land-based hatchery for research. | NA | NA | NA | The broodstock would be translocated to a land-based hatchery. Risks would be translocation of disease and marine pests. These have been covered. However any future hatchery genetic research on mussels and the potential outcomes would require a review of genetic risks, particularly if the intent was to grow selectively bred or sterile progeny on mussel farms. Current and proposed translocation practices of the mussel industry do not constitute a high genetic risk for mussels. A section has been added to the report on translocation of mussels for research purposes. |
Appendix 2. Functional Groups for Marine Pests
Key unwanted species divided into functional groups. Modified from the functional group list developed for the Marine Pest Monitoring Manual (Anon, in press). X = present in the functional group. Water (W) = Water transported in association with mussel adults (i.e. with closed shells), spat and associated gear. Sediment (S) = Soft substrate e.g. sand, Hard Surface (H) = hard substrate such as the gear (ropes, anchors) and mussels themselves.
| Key unwanted species | Functional group | Medium Water (W), Sediment (S), Hard Surfaces (H) | |||||
|---|---|---|---|---|---|---|---|
| Sessile Fouling (Hard) | Sessile Infauna (Soft) | Motile (soft) | Motile (hard) | Meroplanktonic | Holoplanktonic | ||
| Alexandrium minutum1 (toxic dinoflagellate) | X | X | W, S | ||||
| Asterias amurensis2 (North Pacific seastar) | X | X | X | X | W, H | ||
| Vancorbula gibba2 (European clam) | X | X | W, S | ||||
| Crassostrea gigas2 (Pacific oyster) | X | X | X | W, S, H | |||
| Gymnodinium catenatum1 (toxic dinoflagellate) | X | X | W, S | ||||
| Mnemiopsis leidyi (sea walnut) | X | W | |||||
| Potamocorbula amurensis1 (Asian clam) | X | X | W, S | ||||
| Sabella spallanzanii2 (giant fanworm) | X | X | X | W, S H | |||
| Undaria pinnatifida2 (Japanese seaweed) | X | ? | X | W, H | |||
| Carcinus maenas2 (European shore crab) | X | X | X | W, H | |||
| Musculista senhousia2 (bag mussel) | X | X | X | W, S, H | |||
Appendix 3. Toxic Algae Alert Levels Specified in the Victorian Shellfish Quality Assurance Program
Phytoplankton Abundance Triggers for the VSQAP (cells/L) (Department of Environment and Primary Industries (2004))
| Alga / Algal Group | Toxin | Warning | Tissue Testing | Harvest Suspension Pending Toxin Analysis | Harvest Resumption |
|---|---|---|---|---|---|
| Bacillariophyceae | |||||
| Pseudo-nitzschia spp. (<50% total phytoplankton) | ASP (domoic acid) | 100,000 | 300,000 | 500,000 | <20 µg/g domoic acid for three successive samples over 14 days; phytoplankton abundance not rising. |
| Pseudo-nitzschia spp. | |||||
| (>50% total phytoplankton) | ASP | 50,000 | 100,000 | 200,000 | As above |
| Rhizosolenia cf chunii | Bitter Taste | 10,000 | N/A | 20,000 Level 2 Warning | Harvesting suspended/resumed by growers depending on taste of mussels. |
| Dinophyceae | |||||
| Alexandrium catenella | PSP | 100 | Routine or 100 | *500Dz | <80 µg/100g PSP for three successive samples over 14 days; phytoplankton abundance not rising. |
| Alexandrium minutum | PSP | 100 | Routine or 100 | *500 | As above |
| Alexandrium tamarense | ?PSP | 100 | Routine or 100 | *500 | As above |
| Alexandrium spp. (unknown or in doubt) | ?PSP | 100 | Routine or 100 | *500 | As above |
| Dinophysis acuminata | DSP | 1,000 | 1,000 | 2,000 | <20 µg/100g DSP for two successive samples taken not <7 days apart; phytoplankton abundance not rising. |
| Dinophysis caudata | DSP | 1,000 | 1,000 | 2,000 | As above |
| Dinophysis fortii | |||||
| Dinophysis spp. | ?DSP | 1,000 | 1,000 | 2,000 | As above - precautionary only till further information available. |
| Gymnodinium catenatum | PSP | 100 | Routine or 100 | *500 | <80 µg/100 g PSP for three successive samples over 14 days; phytoplankton abundance not rising. |
| Karenia mikimotoi Karenia brevis Karenia cf brevis (Flat, Australian species morphologically similar to K. brevis). (K. brevis probably not present in Aust) | NSP brevetoxin (BTX) | 1,000 | 2,000 | 5,000 | <20 mouse units/100g for two successive samples taken not <2 days apart; phytoplankton abundance not rising. |
| Prorocentrum lima | ?DSP | 1,000 | 1,000 | 2,000 | <20 µg/100g DSP for two successive samples taken not <7 days apart; phytoplankton abundance not rising. |
* Draft Model National Marine Biotoxin Management Plan trigger adopted until more information on DTX 3 (OA esters) is available for PPB; PTX-2SA are no longer included as a toxin.
NB: Harvest suspension pending biotoxin analysis is precautionary; resumption of harvesting will be determined on the basis of toxin levels.
Appendix 4. Notifiable Molluscan Diseases Listed by the Victorian Fisheries Regulations 1998 - Schedule 16
Scientific name and common names of notifiable diseases in molluscs.
| Disease Organism | Disease |
|---|---|
| Bonamia ostreae | Bonamiosis |
| Bonamia spp. | Bonamiosis |
| Haplosporidium amoricanum | Haplosporidiosis |
| Haplosporidium costale | Haplosporidiosis |
| Haplosporidium nelsoni | Haplosporidiosis |
| Marteilia refringens | Marteiliosis |
| Marteilia sydneyi | Marteiliosis |
| Mikrocytos mackini | Mikrocytosis |
| Mikrocytos roughleyi | Mikrocytosis |
| Iridovirus | Oyster velar disease |
| Perkinsus atlanticus | Perkinsosis |
| Perkinsus marinus | Perkinsosis |
| Perkinsus olseni | Perkinsosis |
Appendix 5. Hatchery Compliance Report
Hatchery Compliance Report for Use in the Authorisation of Translocation of Mussel seed into Victoria
Hatchery Check List
Hatchery Name:
_________________________________________________________
Hatchery address:
_______________________________________________________
Requested Examining Officer:
_____________________________________________
Name: ____________________________________________
Title: _____________________________________________
Date of visit: ____________________________________________________________
PLEASE CIRCLE EITHER YES OR NO
1. On the day of this inspection no other marine mollusc species excluding mussels were seen on the premises (e.g. oyster, scallop and abalone).
YES NO
Comments:_____________________________________________________________
______________________________________________________________________
_________________________________________________
2. A sample of the mussel broodstock to be spawned has been sent to an authorised veterinary agency for a health examination.
YES NO
Comments:_____________________________________________________________
______________________________________________________________________
______________________________________________________________________
3. Broodstock have been scrubbed and cleaned.
YES NO
If answered NO, then hatchery manager advises they will do so before spawning.
YES NO
Comments:_____________________________________________________________
______________________________________________________________________
__________________________________________________________________
4. Hatchery manager indicates the source of broodstock as coming from:
Site:
_______________________________________________________________________
Lease #:
___________________________________________________________________
Lease owner:
____________________________________________________________
Comments:______________________________________________________________
_______________________________________________________________________
_________________________________________________________________
5. Are all hatchery staff aware of the requirements in this report?
Staff Members Names
1. _____________________________YES NO
2. _____________________________YES NO
3. _____________________________YES NO
4. _____________________________YES NO
5. _____________________________YES NO
Comments:_______________________________________________________________
________________________________________________________________________
__________________________________________________________________
6. Larvae and nursery tanks appear clean and free of cracks or de-laminating on inner surface.
(Examine all larvae and nursery tanks visually from the top of tank for any obvious cracks or de-laminating.)
YES NO
Comments:________________________________________________________________
_________________________________________________________________________
7. Hatchery manager advises that larval tank valves have been dismantled, scrubbed and cleaned with
chlorine or similar cleaning agent.
YES NO
Comments:__________________________________________________________________
___________________________________________________________________________
_________________________________________________________________
8. Get hatchery staff to dismantle two randomly selected valves on the larval tanks for examination.
Are the valves clean (i.e. no shellfish spat observed on inner valve surfaces and no smell of decaying organic matter)?
YES NO
Comments:____________________________________________________________________
_____________________________________________________________________________
__________________________________________________________________
9. Larval grading screens and associated tubes and pipes are observed to be clean and free of larvae and spat.
YES NO
Comments:_____________________________________________________________________
______________________________________________________________________________
_________________________________________________________________
10. All larvae and nursery systems use seawater filtered to less than 50 micron.
YES NO
Comments:_______________________________________________________________________
________________________________________________________________________________
_________________________________________________________________
11. Primary seawater filtration system Hatchery Management advises that:
Sand or other filter systems are well maintained and working to remove particles to a less than 50 micron.
YES NO
Any header tanks are clean YES NO
System is back-flushed regularly YES NO
12. Secondary filtration system (filter bags or similar system)
Hatchery Management advises that:
Filters are in place on header tank. YES NO
Filters are available for larval & setting tanks. YES NO
Will filters be used on larval and setting tanks? YES NO
Sufficient filters are on hand. YES NO
Comments:__________________________________________________________________________
___________________________________________________________________________________
_________________________________________________________________
13. Hatchery management advises that cultch used for the setting of mussels is clean and if second hand has been sourced from Victorian farms.
YES NO
Comments:___________________________________________________________________________
____________________________________________________________________________________
_________________________________________________________________
14. Mussel setting ropes appear to be new or clean.
YES NO
Comments:___________________________________________________________________________
____________________________________________________________________________________
_________________________________________________________________
Signature of delegated officer:
___________________________________________
Title of delegated officer:
________________________________________________
Please print name:
______________________________________________________
Date:
Signature of hatchery manager:__________________________________________
Please print name: ____________________________________________________
Date: _______________________________________________________________
Appendix 6. Information to Conduct a Risk Assessment for Translocating Mussels from Interstate into Victoria
Most background information required to conduct a risk assessment to allow the translocation of mussels into Victoria can be found in Fishing and Aquaculture on the Department of Environment Primary Industries website: www.depi.vic.gov.au
Useful background documents and specific application forms and guidelines are listed below:
- Anon. 2004. An Introduction to Translocations of Live Aquatic Organisms in Victoria. Fisheries Note FN169. State of Victoria. Department of Environment and Primary Industries.
- Anon. 2003. Guidelines for Assessing Translocations of Live Aquatic Organisms in Victoria. State of Victoria. Department of Environment and Primary Industries.
- Gunthorpe L, Mercer J, Rees C, and Threodoropolous T (2001) Best practices for the sterilisation of aquaculture farming equipment: A case study for mussel ropes. Marine and Freshwater Resources Institute Report No 41.
- 4. Anon. Initial Screening Application for the translocation of live aquaticorganisms in Victoria. State of Victoria. Department of Environment and Primary Industries.
- Anon. Risk Assessment Proforma and Instructions for the Translocation of Live Aquatic Organisms. State of Victoria. Department of Environment and Primary Industries.
