Environmental Monitoring of Marine Aquaculture
MAFRI Report No. 46. (2002)
A Review of Appropriate Methods
Fiona Gavine and Lachlan McKinnon
December 2002
ISSN:1328 - 5548
ISBN:1 74106 738 3
Preferred Way To Cite This Publication:
Gavine, F. M. and Mc Kinnon, L. J. 2002. Environmental Monitoring of Marine Aquaculture in Victorian Coastal Waters: A Review of Appropriate Methods. Technical Report No. 46. Marine and Freshwater Resources Institute, Victoria.
Glossary
Aquaculture - Marine - Marine aquaculture (or mariculture) Marine aquaculture is the cultivation and harvesting (or farming) of fish, shellfish and other aquatic species, including seaweeds, utilising seawater as a growing medium
Aquaculture - Technologically established - Aquaculture species, which have closed production, cycles (hatchery-bred broodstock and seed) and commercially produced pelleted diets.
Aquaculture -New and developing - Species are those which source seedstock or broodstock from the wild and/or feed trash fish or non-specific diets.
Avian predator - Hunting bird.
Bathymetry - The measurement of ocean or lake depth and the study of floor topography.
Benthos - Organisms which inhabit the seabed.
Catch quota - A limit of fish caught used by professional fisherman to ensure sustainability of species.
Depuration - To make free from impurities, cleanse by placing in clean water.
Detritus - Small pieces of dead and decomposing plants and animals
Eh - Redox potential
Endemic - Native to a particular area and found nowhere else in the world.
Environmental carrying capacity - Amount of load an environment or ecosystem can handle.
Epifauna - Animals living on the surface of the ocean bottom.
Eutrophication - Over enrichment of a water body with nutrients, resulting in excessive growth of organisms and depletion of oxygen concentration.
Fallow - Method of allowing land to lie for a season or more in order to increase in its productivity or to allow it to recover from the stresses of cultivation.
Food conversion ratio - Amount of food required to produce one unit of growth in an organism.
Generic hazard assessment - Hybrid - Offspring of a cross between genetically dissimilar individuals.
Hydrography - The science of the measurement, description and mapping of the surface waters of the earth with special reference to their use for navigation.
in situ - In it's original place.
Macrobenthic - The larger organisms of the benthos, exceeding 1mm in length.
Mooring system - Series of ropes, chains, weights and anchors used to keep longlines cages or nets in place in the ocean or lakes.
Nitrogen cycling - The biochemical cycle of nitrogen involving fixation, nitrification, decomposition by putrefaction and denitrification.
Offshore culture - The aquaculture production of aquatic animals out at sea or in large lakes using cages or longlines.
Pathogen - Any disease producing microorganism.
Phototrophic - Requiring light as a source of energy in nutrition.
Polychaetes - A class of annelids, commonly called bristle worms.
Ranching - A form of aquaculture where juveniles are released into the wild, allowed to grow and released at a later date.
Regular monitoring - Periodic recording of a set of parameters.
Sedentary organisms - Organisms which are attached to the substrate and not free living
Sediment-water interface - The contact surface between sediment and water.
Spatfall - The adhesion of spawn of bivalve molluscs onto objects beneath the water.
Substrate - A hard surface to which something attaches
Executive Summary
The Victorian marine aquaculture industry had a farm gate value of approximately $2.8 million in 2000/01 and is relatively small compared with other Australian states. The industry is currently dominated by the production of bivalve shellfish, primarily blue mussels (Mytilus edulis) although there has been considerable capital investment in abalone growout in recent years.
The environmental impact of marine aquaculture is an important issue for the future development of the industry. It is important that the environmental effects of marine aquaculture are managed in a manner that is acceptable to the broader community. An appropriate environmental monitoring regime and remedial actions are key components to managing the environmental effects of marine aquaculture.
The purpose of this review is to provide a scientific basis and recommendations for the development of an appropriate environmental monitoring regime for marine aquaculture in Victoria. This document specifically addresses environmental issues related to offshore culture of shellfish (e.g. mussels, scallops, oysters) and fin fish (e.g. salmonids, kingfish and snapper). It is the view of the authors that these two activities cover the spectrum of environmental effects arising from marine off-shore aquaculture. Bivalve shellfish farming relies on natural productivity with a net removal of nutrients from the water column. Finfish culture is usually an intensive industry which involves a net addition of solids and nutrients to the marine environment.
The review identifies the environmental issues associated with marine aquaculture and appropriate techniques for monitoring and remediation of those environmental impacts through a comprehensive review of the literature and international and interstate management practices. A generic risk/hazard assessment was undertaken to categorise and prioritise issues. Actual environmental risks associated with marine aquaculture development, and the associated monitoring and management of those risks, was found to be site specific depending on the species, location (characteristics and sensitivity), culture system and husbandry methods employed.
Finally the review recommended an environmental monitoring and management regime for marine aquaculture developments in Victorian waters. The monitoring and management strategy developed was primarily based on the experience of Tasmania and South Australia, adapted to suit Victorian legislative requirements. The review recommended that the environmental management of offshore aquaculture development should proceed in three phases, viz:
- Characterisation studies for marine aquaculture areas. This should provide a "broadbrush" summary of the various physical and ecological attributes within the aquaculture zone. It should identify areas sensitive to aquaculture development, potential risks to aquaculture operations and recommend appropriate species, production methods, intensity of production and management controls at the zone.
- Development of licence conditions for marine aquaculture. The review recommended appropriate licence conditions for marine aquaculture in Victoria that allowed for a combination of self-monitoring and reporting by the industry which is underpinned by a Government monitoring compliance strategy. Full details of licence conditions for lease holders in each area will be determined in the management planning process.
- Monitoring Requirements for Aquaculture Licence Areas. The review recommended that monitoring be carried out at each licence area before production begins (Initial Monitoring/ Baseline Survey) and also at specified intervals once the site was operational (Routine Monitoring).
The aim of the Initial Monitoring survey should be to provide a reference data set which can be used to establish the conditions of the local ecosystem against which future change can be compared. Initial monitoring should be carried out for:
- existing shellfish aquaculture sites
- new shellfish aquaculture sites
- finfish aquaculture sites.
The recommended parameters for the Initial monitoring surveys are shown below. Specific monitoring protocols will be developed for each area as part of the management planning process.
| Shellfish | Finfish | |||
|---|---|---|---|---|
| Existing | New | New | ||
| Bathymetric survey | ✔ | ✔ | ✔ | |
| Hydrographic survey | ✔ | |||
| Video | ✔ | ✔ | ✔ | |
| Seabed characteristics/ Important habitat profile | ✔ | ✔ | ✔ | |
| Sediment Chemistry | ||||
| Visual | X | ✔ | ✔ | |
| Redox | X | ✔ | ✔ | |
| Organic content (C/N) | X | ✔ | ✔ | |
| Stable isotope analysis | X | ✔ | ✔ | |
| Particle size analysis | X | ✔ | ✔ | |
| Sediment Biology | ||||
| Biological/ benthos | X | ✔ | ✔ | |
✔= required; X = required if triggers exceeded
Regular monitoring strategies for shellfish and finfish aquaculture at each licence area were also recommended by the review. Specific monitoring protocols will be developed for each areas as part of the management planning process. In the case of shellfish monitoring a video transect survey will be required annually and further monitoring required if specified trigger levels are exceeded. More stringent and frequent monitoring is required for finfish aquaculture.
| Shellfish | Finfish | ||||
|---|---|---|---|---|---|
| Frequency | Frequency | ||||
| Video | ✔ | Annual | ✔ | 6 Month | |
| Sediment Chemistry | |||||
| Visual | X | If required | ✔ | Annual | |
| Redox | X | If required | ✔ | Annual | |
| Organic content (C/N) | X | If required | ✔ | Annual | |
| Stable isotope analysis | X | If required | ✔ | Annual | |
| Particle size analysis | X | If required | ✔ | Annual | |
| Biological | ✔ | ||||
| Biological/ benthos | X | If required | ✔ | Annual | |
✔ = required; X = required if triggers exceeded
1. Introduction
Globally, the aquaculture industry has developed rapidly over the past two decades in response to the stagnation of fish production from traditional capture fisheries (Figure 1.1), the inexorable rise in the world's population (FAO, 2000) and the resulting increasing demand for animal protein.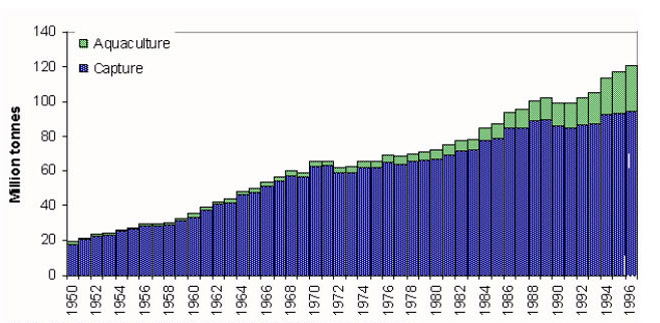
Note: Aquaculture quantities prior to 1984 are provisional
Figure 1.1: Aquaculture and capture fisheries production 1950-1996.
Aquaculture, as an industry, has the unique potential to make up the shortfall in protein supply for human consumption by the production of marine and freshwater aquatic species. Recently, some authors have suggested that some forms of aquaculture (specifically carnivorous finfish) actually increase pressure on oceanic fisheries through their dependence on fishmeal made from wild fish (Naylor et al 2000). However it should be noted that 93% of fishmeal is currently utilised in terrestrial agriculture and aquaculture use only accounts for 4% (Gill, 1997). In addition, the fish used to produce fishmeal are small pelagic fish, which are generally unsuitable for direct human consumption (Welcomme, 1996).
Aquaculture is a relatively new industry world-wide and suitable species and culture technologies are continually being developed and refined to ensure the environmental and economic sustainability of the industry. Many of the environmental issues that arose during the 1980's and 1990's in Europe, USA and Asia (e.g. disease problems and environmental degradation) damaged public perception of the industry and directly affected the economic viability of commercial aquaculture. These issues had to be addressed by both aquaculturists and Government agencies to ensure the long-term sustainability of the industry. Management protocols have subsequently been developed for most culture systems and species to minimise or eliminate potential environmental impacts (e.g. EPA, 1999 for the Victorian trout industry; APFA, 1998 for the prawn farming industry). In addition, formal environmental management systems have been developed to integrate best practice environmental management into daily aquaculture operations (e.g. Gavine et al 1996). This development and refinement of technologies and management practices will continue to occur as the economic viability of intensive aquaculture (particularly marine finfish and shellfish culture) is intrinsically linked to the maintenance of good environmental quality.
1.1 Industry Status In Australia
The marine aquaculture industry in Australia is developing rapidly in all States and Territories and in 1999/00 produced nearly 37,000 tonnes of product with a farm gate value of $695 million (O'Sullivan and Dobson, 2001). Aquaculture production has increased three-fold since the start of data collection in 1988/89 and the value has increased by 500%. The most important marine and estuarine species in terms of production and value in Australia are shown in Table 1.1. The pearl oyster (Pinctada maxima) industry is the most valuable in Australia, however, levels of production are not known as the species is grown for it's pearl rather than the meat. The most valuable species by far of marine finfish are southern bluefin tuna (fattening) and Atlantic salmon, both farmed in the southern latitudes of Australia. The development of abalone farming will further increase the value of the industry over the next 2-3 years due to the high market value of this product ($35-40/kg) and the large number of juveniles currently being grown out.
Table 1.1: Marine aquaculture production in Australia 1999/00 (O'Sullivan and Dobson, 2001).
| Species | Production (tonnes) |
Value ($000's) |
|
|---|---|---|---|
| Finfish | |||
| Atlantic salmon | 7,257 | 66,324 | |
| Barramundi | 879.6 | 8,860.6 | |
| Southern Bluefin Tuna | 7,780 | 202,000 | |
| Crustaceans | |||
| Marine prawns | 2,705 | 41,068 | |
| Molluscs | |||
| Pacific oysters | 5,005 | 24,073 | |
| Sydney rock oysters | 5,106 | 29,115 | |
| Blue mussels | 2,006 | 5,561.2 | |
| Pearl oysters | Nda | 245,500 | |
Compared with other states and territories, the Victorian marine aquaculture industry is relatively small (<10% of the total) and dominated by the culture of blue mussels (Mytilus edulis). The total Victorian mussel production in 1999/00 was 540 tonnes with a farm-gate value of $1.5 million (O' Sullivan and Dobson, 2001). Abalone aquaculture has been developing in Victoria over the past 5 years, with the creation of 7 land-based and 6 off-shore private aquaculture ventures. Blacklip abalone (Haliotis rubra) stock currently laid down in ongrowing systems is valued at $10 million with a forecast of $20 million production by 2005. The commercial culture of greenlip abalone (H. laevigata) is also currently being investigated. Other shellfish species with potential for aquaculture in Victoria are scallops (Pecten fumatus, Equichlamys bifrons, Chlamys asperrimus) and oysters (Ostrea angasi, Pinctada imbricata). There is currently no commercial production offshore of marine finfish in Victoria, but the potential for development of this industry is good given the coastal resources of the state.
In August 2000, the Environment Conservation Council (ECC) completed its investigation of the coastal resources of Victoria and recommended the creation of 12 marine aquaculture zones (Table 1.2), including two land based zones, with a total area of 2,682 ha, including 983 ha of currently farmed area (ECC, 2000). The recommended areas were subsequently approved by Parliament with the passing of the National Parks (Marine Parks and Marine Sanctuaries) Act in June 2002. The next step in development of the aquaculture zones is to prepare a management plan that specifies the operational, environmental and administrative requirements for the zone, as specified in the Fisheries Act 1995. Following the development of management plans, Fisheries Victoria will allocate lease areas within each zone to current or prospective aquaculture producers.
Table 1.2: Recommended aquaculture zones in Victorian coastal waters (ECC, 2000).
| Number | Zone | Geographic Area | Area | Landbased / Offshore |
New / Existing / Expanded |
|---|---|---|---|---|---|
| E1 | Portland | Portland Bay | 200 ha | Offshore | New |
| E2 | Grassy Point | Port Phillip Bay | 252 ha | Offshore | Existing/Expanded |
| E3 | Clifton Springs | Port Phillip Bay | 315 ha | Offshore | Existing |
| E4 | Pt Lillias | Port Phillip Bay | 40 ha | Land-based | New |
| E5 |
Avalon |
Port Phillip Bay | 17 ha | Land-based | Existing |
| E6 | Bates Point | Port Phillip Bay | 25 ha | Offshore | New |
| E7 | Kirk Pt- Werribee | Port Phillip Bay | 200 ha | Offshore | New |
| E8 | Beaumaris | Port Phillip Bay | 25 ha | Offshore | Existing/Expanded |
| E9 | Mount Martha | Port Phillip Bay | 150 ha | Offshore | Existing/Expanded |
| E10 | Dromana | Port Phillip Bay | 20 ha | Offshore | Existing/Expanded |
| E11 | Pinnace Channel | Port Phillip Bay | 1000 ha | Offshore | New |
| E12 | Flinders | Westernport Bay | 440 ha | Offshore | Existing/Expanded |
The Victorian marine aquaculture industry has huge development potential to capitalise on the high value of marine finfish and shellfish. However, it is vital that this development is regulated and managed in an environmentally sustainable manner to protect both the fish farmer and the marine environment from detrimental impacts. The potential environmental impacts of aquaculture are well documented following comprehensive research studies in various parts of the world (see NCC, 1989; Barg, 1992 for summaries). Victoria has much to learn from the experiences of other states and countries and is well placed to adopt the regulatory approaches, monitoring strategies and management protocols developed in response to environmental issues which have faced the industry in the past but have been successfully addressed and managed.
1.2 Purpose
The purpose of this review is to provide a scientific basis and recommendations for the development of an appropriate environmental monitoring regime for marine aquaculture in Victoria. This document specifically addresses environmental issues related to offshore culture of shellfish (e.g. mussels, scallops, oysters) and fin fish (e.g. salmonids, kingfish and snapper). It is the view of the authors that these two activities cover the spectrum of environmental effects arising from marine off-shore aquaculture.
Monitoring and management of effluent from land-based marine aquaculture facilities are subject to existing NRE Fisheries and EPA regulations. This document reviews current environmental monitoring regimes for offshore marine aquaculture and makes recommendations, based upon existing Best Practice, for the environmental monitoring for marine aquaculture in Victorian coastal waters.
Specifically, the aims of this document are to:
- Characterise the production methods of shellfish and finfish marine aquaculture industries which have potential for development in Victoria;
- Summarise the potential impact of offshore shellfish and finfish aquaculture on the marine environment through a review of relevant scientific literature;
- Review environmental monitoring techniques recommended for aquaculture;
- Review various approaches to regulating and monitoring the industry both interstate and overseas; and
- Recommend appropriate environmental monitoring strategies for marine aquaculture in Victoria.
2 Marine Aquaculture Production Methods
As previously outlined the main forms of marine aquaculture production systems that have potential for development in Victoria are:
- Shellfish culture – which includes bivalve mollusc culture (mussels, scallops, pearl and edible oysters) and gastropod mollusc culture (abalone);
- Marine finfish culture – which includes "technologically established1" finfish aquaculture species (salmonids) and "new and developing" finfish aquaculture species (e.g. yellowtail kingfish, Southern bluefin tuna, snapper).
2.1 Shellfish Culture
2.1.1 Bivalve mollusc culture
Bivalve molluscs have been commercially cultured in Victorian waters for over 20 years. The predominant culture method for bivalve shellfish is using "long lines" where the molluscs are suspended from ropes above the seabed (Figure 2.1). This form of culture has the advantage of minimising predation of the species under culture by sedentary or other organisms, reducing competition for feed by other filter feeders, and usually results in a cleaner, thinner-shelled product. In Victoria, farms generally have a total of 8-10 longlines (100-120 m in length) per 3 ha licensed area. "Mussel droppers" are hung vertically from the longline at regular intervals, 40 cm intervals in Port Phillip Bay, 80 cm intervals in Western Port Bay. This reflects the higher wave action in Western Port as well as the lower productivity of the water. Farmers culture between 225 and 300 droppers per longline in Port Phillip Bay and 110 and 150 droppers in Western Port Bay.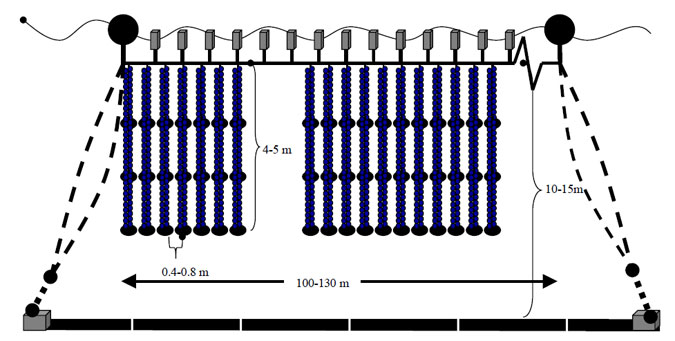
Figure 2.1: Side view of mussel longline at showing attached lines, floats and weights (courtesy of John Mercer, DNRE).
1 "Technologically established" aquaculture species in this context is defined as those which have closed production cycles (hatchery-bred broodstock and seed) and commercially produced pelleted diets. Conversely "new and developing" species are those which source seedstock or broodstock from the wild and/or feed trash fish or non specific diets.
Each dropper will produce between 15 and 30 kg of mussels over a production cycle, which is usually between 12-18 months. The mussel industry in Victoria has a year-round culture season, with maximum product availability from July to January.
Bivalve molluscs are filter feeders and are dependent on phytoplankton, bacteria and particulate organic matter in the water column for their nutrition. Molluscs have the capacity to filter large volumes of the surrounding seawater. For example, Jorgensen (1952) reported that at between 22 - 23°C the pumping rate of the oyster C. virginica was 0.5 - 0.8 litres per hour per g wet weight of flesh (mean 0.54 litres per hour). Using this data, a standard Victorian mussel lease with 10 longlines and 250 droppers per longline, with a maximum biomass of 20 kg per dropper, would filter around 259 m3 per day at maximum stocking density (assuming a meat: total weight ratio of 40%).
Bivalves are ideally cultured in waters which contain relatively high nutrient levels and can sustain healthy phytoplankton populations. Blue mussels ideally require water temperatures between 12- 26oC and salinity range between 20 and 37 ppt (ASIC, 1997). The culture area should have high dissolved oxygen concentrations and low sediment loads, and with water currents at least 5 cm/s (ASIC, 1997). The culture area should be relatively sheltered to reduce stock loss from wind and wave action. Site selection is extremely important for bivalve mollusc culture. Bivalves grown in contaminated areas can concentrate pollutants, particularly bacteria, heavy metals and other toxins, which may lead to problems with product quality. In particular, water quality problems resulting from waste sources such as sewage and industrial waste outfalls or spills can make bivalves unsuitable for consumption. The concentration of bacteria, virus particles and toxins due to algal blooms (eg Dinophysis spp.), in bivalve molluscs has particularly adverse implications for commercial bivalve culture. NRE Fisheries, together with industry, operates the Victorian Shellfish Quality Assurance Program (VSQAP), which serves to monitor and manage the industry based on product and environmental quality.
2.1.2 Abalone aquaculture
Abalone (Haliotis sp.) are marine gastropods which are endemic to Australia. There are also many species of abalone found in Victorian waters with two being harvested or cultured commercially: Haliotis laevigata (greenlip) and Haliotis rubra (blacklip) (Hone and Fleming, 1997). A hybrid of these two species is also being cultured.
In the wild, these species of abalone are found in cool (10-24°C), turbulent waters and their preferred depth is 5-30m. In culture systems their growth is slow, reaching market size of 70 mm in 2.5-3.5 years and 145 mm in 4-5 years. They may be commercially cultured in land-based tank systems, or at sea in cages. Greenlip abalone is considered the best for tank culture as it has a faster growth rate (20-30 mm/yr) compared to blacklip (15-20 mm/yr). The optimum temperature for culture is 15-18°C and salinity 34-37 ppt. Abalone are herbivorous and feed on microalgae and coralline algae in juvenile stages and macroalgae when older. Commercially produced compound feeds have now been developed and are predominantly composed of carbohydrate and protein (including semolina, soyflour, fishmeal and casein). Food conversion ratios (FCRs) of 1.3-1.5:1 have been obtained using commercial diets (Fleming and Hone, 1996). When fed on fresh seaweed abalone are less efficient feeders with FCRs based on a wet weight of 20-30:1. The difference between land-based abalone aquaculture and cage culture in open waters is the intensity of the operation. Land-based culture is generally semi-intensive to intensive as a higher production is required to cover the high capital and operational costs. Cage culture however is extensive or semi-intensive with cages (or barrels) positioned on the seafloor or suspended from longlines. Stocking densities in cage culture units are lower than on land and growth rates will be slower due to variations in ambient temperatures. Abalone may be fed supplementary diets in cages, but may also be left to rely on natural productivity.
2.2 Finfish culture
Cage culture is widely used both interstate and overseas as a cost-efficient method of finfish production. A wide variety of cage designs are now commercially available but the common features are a floating collar, a suspended net bag and a mooring system (Figures 2.2 and 2.3).
A summary of cage culture in Australian marine and inland waters is provided by Gooley et al. (2001). There is currently no commercial cage culture of finfish in Victoria, but the potential for development of this industry is significant. The main species that could be considered in the ambient climatic conditions are salmonids such as Atlantic salmon (Salmo salar), rainbow trout (Oncorhynchus mykiss), brown trout (S. trutta), southern bluefin tuna (Thunnus maccoyii), snapper (Pagrus auratus) and yellowtail kingfish (Seriola lalandi). The choice of species will have important consequences in relation to site selection and environmental impacts as "technologically established" aquaculture species, such as Atlantic salmon, use commercial pelleted diets which are readily accepted and very efficiently converted by the fish. "New or developing" species such as southern bluefin tuna are fed largely on a diet of fresh or frozen pilchards, which are less efficiently converted by the fish and consequently produce more waste. Manufactured diets for this species are currently being trialed in South Australia
"Technologically Established" Finfish Aquaculture (e.g salmon aquaculture in Tasmania)
The Atlantic salmon industry developed as a joint venture between a group of Australian companies and a Norwegian company (Noraqua) in the mid 1980's. Since then the industry has expanded to a production of greater than 7,000 tonnes per annum and could provide a template for sustainable marine aquaculture development in Victoria. The Atlantic salmon brood fish spawn in freshwater and continue to grow in freshwater for 8-15 months before being transferred to sea cages as smolts from Autumn to Spring. Smolts range in size from 60-200g. The aim of the growout cycle is to harvest fish all year round so smaller, slower growing fish will be ready for harvest later than the "shooters". In the sea cages salmon are initially stocked at densities of <1 kg/m3 and reach a maximum at harvest of 12-15 kg/m3. This stocking density is low compared with those used overseas. The fish are fed commercial pelleted diets and consistently achieve FCRs of 1.4-1.5:1. This has reduced from 1.8-2.0:1 over the past 10 years through the development of better diets (DPIWE, 1999). Salmon are harvested from sea cages after one year at a weight of approximately 4 kg.


"New Species" Aquaculture (e.g. southern bluefin tuna in South Australia)
The southern bluefin tuna (Thunnus maccoyii) industry began on the Eyre Peninsula in South Australia in the early 1990's and is essentially a fish "ranching" exercise. Entire schools of wild tuna (15-20 kg) are caught in the southern ocean using the purse seine method and transferred to floating pontoons that are towed back to shore (Napier, 1999). The fish are then transferred to floating cages up to 50 m in diameter where they are held for 3-6 months until they reach market size (30-40kg) (PIRSA, 1999). Tuna farmers and fishermen are subject to a strict wild catch quota system, which currently stands at 5,625 tonnes per annum. Of this, 4,500 tonnes are currently farmed (PIRSA, 1999) and with an increase of up to 100% in body weight over the culture cycle this allows an extra tonnage of tuna to be produced over the catch quota. The value of the tuna aquaculture industry in South Australia soared to around $202 million in 1999/00. Ranched tuna are fed with pilchards, 50% of which are imported from northern California and 50% are caught locally; FCRs typically average 15:1. Stocking density is restricted by licence at 4 kg/m3. Research is currently underway to produce a commercial pelleted diet as reliance on wild caught stocks is risky from both a supply and disease transfer perspective.
3 Environmental Issues Related to Marine Aquaculture
Finfish and shellfish farming can both impact on the marine environment, although in different ways. Finfish culture is usually an intensive industry which involves a net addition of solids and nutrients to the marine environment, whereas shellfish are predominantly farmed using techniques which rely on natural productivity with a net removal of nutrients from the water column (NCC, 1989). The exception to this is abalone culture (gastropods) where artificial diets are often used in culture practices. The environmental issues associated with each marine aquaculture system are summarised below. A generic risk/hazard assessment was carried out on the issues identified using the methodology shown in Appendix I. This hazard assessment is based on the literature reviewed as part of this exercise and the experience of the authors in monitoring the impacts of aquaculture on the environment. Actual risks associated with marine aquaculture development will be site specific depending on the species, location (characteristics and sensitivity), culture system and husbandry methods employed. Site specific risks will be quantified and evaluated as part of the Management Plans to be developed for each aquaculture area.
3.1 Shellfish Culture
3.1.1 Bivalve mollusc culture
Compared with intensive finfish culture, environmental concerns associated with bivalve mollusc culture are low and normally only occur where culture covers a large area, have very high stocking densities, or are not properly managed. Figure 3.1 shows the flow of nutrients (specifically carbon (C) and nitrogen(N)) through a mussel longline (after Rodhouse et al. 1985). Nutrients in the form of phytoplankton are filtered from the water column by the molluscs and a large proportion (42 % N and 58% C) is excreted. The remaining nutrients are either removed completely from the system during harvest, consumed by scavangers/decomposers or released as solid waste products. Although bivalve mollusc aquaculture is a net remover of nutrients from the ecosystem through harvesting of the product, there is a complex interaction with nitrogen cycling processes in coastal waters.
The main environmental issues related to bivalve shellfish culture in are summarised in Table 3.1. The hazard assessment indicated that the most important environmental concerns were related to accumulation of wastes beneath the culture lines and associated impacts on water and sediment quality. The hazard assessment was carried out with specific reference to bivalve mollusc operations in Victoria where stocking densities are comparatively low and culture areas are restricted to designated aquaculture zones.
Impacts on sediment quality. Impacts on sediment quality may arise from the deposition of solid wastes from the molluscs growing on the longlines. Solid wastes from bivalve culture comprise organic faeces and pseudofaeces, shells and other detritus discarded or dislodged from the farm (NCC, 1989). Studies have shown that a significant proportion of shellfish solid wastes (>90%) are intercepted and consumed by epifauna living on the farm (Tenore et al 1985). The wastes that are deposited will fall through the water column and settle on the sediment beneath or near to the longlines. These wastes can potentially alter the physical character of the sediment, alter nutrient cycling in the sediment or cause biological changes to the macrobenthic community (see 4.1 for more detail).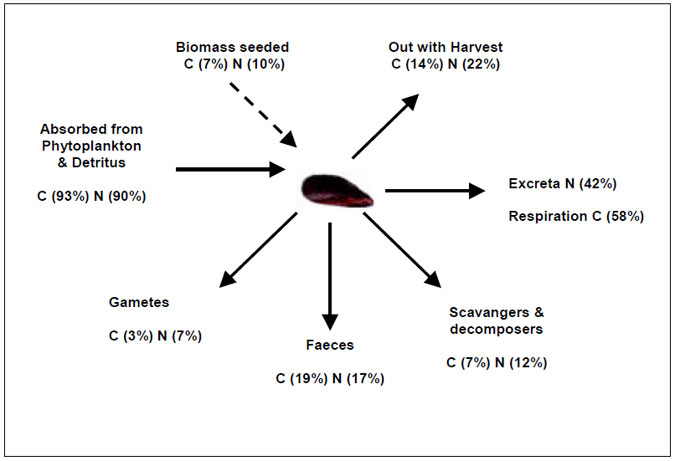
Figure 3.1: Carbon and nitrogen flow though suspended mussel culture (adapted from Rodhouse et al. 1985)
The severity of physico-chemical and biological impacts on sediment quality will depend on the nature of the waste (inorganic or organic) and the extent of waste accumulation, which will in turn depend on water depth, prevailing currents and water movement. Farm management practices also play a role in determining impacts on the sediments. In areas with high densities of shellfish culture and low tidal flushing, this can lead to an accumulation (or concentration) of organic matter in the sediments and the enhancement of benthic fluxes of nutrients (e.g. Souchu et al. 2001).
Kaspar et al. 1985 found that there was an accumulation of organic nitrogen relative to organic carbon and phosphorus in mussel farming sediment and that the ammonium pool in the sediments and rate of in situ denitrification were also higher. Some authors have suggested that the high sedimentation rates in the vicinity of mussel farms may lead to the burial of organic nitrogen and that denitrification in mussel farming areas may be enhanced (Kaspar et al. 1985). This could lead to a decreased availability of nitrogen in areas of high mussel production (Kaspar et al. 1985).
Studies have shown waste accumulation beneath established mollusc farms are strongly influenced by local current patterns (Chamberlain et al 2001). Experience in Tasmania has not found impacts on sediments from shellfish aquaculture to be a significant problem (DPIWE, 1999). In Victoria, benthic processes are known to be particularly important in nutrient cycling within Port Phillip Bay. An important discovery of the Port Phillip Bay Environmental Study is that denitrification in the sediments prevents soluble and available N from accumulating in the water column because it is removed from the main Bay environment in the form of nitrogen gas. In contrast, in some other areas, ammonia, not nitrate is the major N product of organic breakdown. N is returned to the water as ammonia which is available for algal growth. Denitrification efficiencies vary across the Bay and this could have important implications for the capacity of some environments to assimilate waste loadings. Sedimentation of the substrate may prevent the aerobic nitrification process occurring, potentially perpetuating the recycling of ammonia. On a large scale, or in a confined area, this may result in eutrophication. On the scale of marine aquaculture in Victorian waters however, any such impacts on the sediment, should they occur, would be localised to within the aquaculture zone. Such processes would need to occur bay-wide for the risk of eutrophication to occur (Harris et al. 1996).
The hazard assessment showed that sediment impacts were the most important environmental issue related to bivalve shellfish culture in Victoria, although risks are considered moderate and manageable.
Table 3.1: Potential environmental impacts of bivalve mollusc culture (adapted from FAO/NACA, 1995, NCC, 1989).
| Resource | Source | Impact | Potentialconsequences | Hazard Assessment For Victoria |
|---|---|---|---|---|
| Sediments | Metabolic wastes (pseudo faeces). | Accumulation beneath the culture sites. | Localised deterioration in environmental quality. | 6 (moderate) |
| Water column | Filter feeding of stock. | Uptake of primary and secondary production. | Positive impact on coastal eutrophication. | - |
| Depletion of essential nutrients | 4 (low) | |||
| Modification of nutrient cycle | 3 (low) | |||
| Reduction in dissolved oxygen levels. | 3 (low) | |||
| Biological | Seedstock | Collection of wild seed | Impacts on native population? | 3 (low) |
| Stock | Impacts on seagrass beds | Possible competition for feed | 6 (moderate) | |
| Subsurface infrastructure | Habitat creation | Positive impact on biodiversity | - | |
| Cage Infrastructure | Obstruction of native fauna | Potential impacts on whales and dolphins. | 4 (low) | |
| Stock | Creation of shellfish beds | Potential impacts on whales and dolphins. | - | |
| Coastal Resources | Culture infrastructure | Large areas may interfere with the direction and velocity of tidal currents. | Changes in sedimentation patterns. | 3 (low) |
| Navigation | Social conflict | 4 (low) | ||
| Amenity value | Culture infrastructure | Untidy or badly marked | Loss of visual amenity | 5 (low) |
| Servicing sites, processing | Noise, water quality impacts | Loss of amenity | 5 (low) |
Impacts on water quality. Bivalve mollusc culture is not a net contributor of nutrients to the water column as there is no supplementary feeding. Studies have estimated that for each tonne of mussels produced, 32.5 kg carbon, 6.6 kg nitrogen and 0.5 kg phosphorus will be removed from the system (NCC, 1989).
In areas that are not nutrient-enriched, however, the removal of nutrients can lead to nutrient depletion in the surrounding water column. This will have a negative impact on mussel production (and potentially natural biodiveristy) as food will be scarce, so it is important to balance the stocking density at individual sites with the primary productivity or food availability (e.g. MacKenzie, 1998) for both economic and environmental reasons.
Other recorded impacts of bivalve mollusc culture on water quality include modification of the nutrient cycle within coastal ecosystems (NCC, 1989; Rodhouse et al 1985; Kaspar et al 1985) as carbon and nitrogen ingested as phytoplankton will be converted into other forms and concentrated near to the culture area.
The Victorian mussel culture industry stocks at relatively low densities compared with those used overseas and, in addition, only 50% of the zone area is allocated for culture. Impacts on water quality in Victoria will be site specific and a more rigorous assessment will be made for each proposed zone as part of the management planning and allocation process.
Biological impacts. In addition to impacts on plankton and benthic communities outlined above, the collection of seed from the wild is a potential impact on native mollusc stocks, although in reality, impacts are likely to be low as aquaculture collects only a small proportion of the total annual spatfall in Victoria. Ducks are the most likely avian predator on mussel farms in Victoria, but to date have not posed a serious threat to the industry.
Seagrass beds can be impacted by shellfish culture through bio-deposition and loss of stock from the culture lines (ASIC, 1997). Aquaculture infrastructure may also negatively impact seagrass beds and other phototrophic groups through shading and loss of light. In Victoria, the majority of existing shellfish farms and new marine aquaculture areas are sited away from seagrass beds. The existing Clifton Springs Aquaculture Zone is adjacent to seagrass beds, however mussel ropes do not hang directly over seagrass, and are thus unlikely to have detrimental impacts on seagrass communities.
The bivalve culture infrastructure provides new habitat and food chains, on a seasonal basis, for many forms of marine life. Polychaetes and crustaceans in particular are known to thrive in these habitats, which in turn provide a valuable food source for marine fish (N. Hickman, pers. comm.; Holmes, 1982). The creation of beds of shellfish beneath culture ropes may also occur, however the creation of such beds is considered an ecologically and environmentally positive impact. The high abundance and widespread distributions of exotic filter-feeding species may out-compete and displace native species (Cohen et al. 1998), such as mussels, thus the concomitant establishment of mussel beds beneath mussel farms is considered to be of benefit to native mussel populations. The exception to this is in areas where mussel populations are naturally low.
Impacts on coastal resources. Shellfish culture infrastructure can cause resource conflicts if it expands or moves outside of the designated lease area (anchors and moorings can be moved out of position during storms or rough weather). If this occurs, there may be conflict with other users, particularly boat traffic.
Amenity value. Amenity value of the area adjacent to the marine aquaculture lease could be compromised if the aquaculture operation is untidy, unsightly or generates excessive noise, such as may occur during harvesting and maintenance operations. Conversely, there can be positive benefits for recreational fishing as farms may act as both fish attractors and nursery habitats for a variety of commercial and recreational fish species. As a result of this farm sites are often popular sites for recreational fishers.
Other impacts. Other impacts from shellfish culture may be associated with the on-land or indock part of the process. An example of this is wastewater impacts from cleaning the product after harvest. These impacts can be addressed as part of the licence conditions.
3.1.2 Abalone aquaculture
There is very little published information on the environmental impacts of offshore abalone aquaculture, however, since it is generally less intensive than land-based production the related environmental issues are consequently expected to be less significant. The low nutrient content of the feeds, low feeding rate and relatively low stocking densities, growth rate and production, also makes significant adverse impacts on water and sediment quality unlikely. Figure 3.2 shows a mass-balance of nitrogen flow through an abalone culture unit (after Maguire 1988).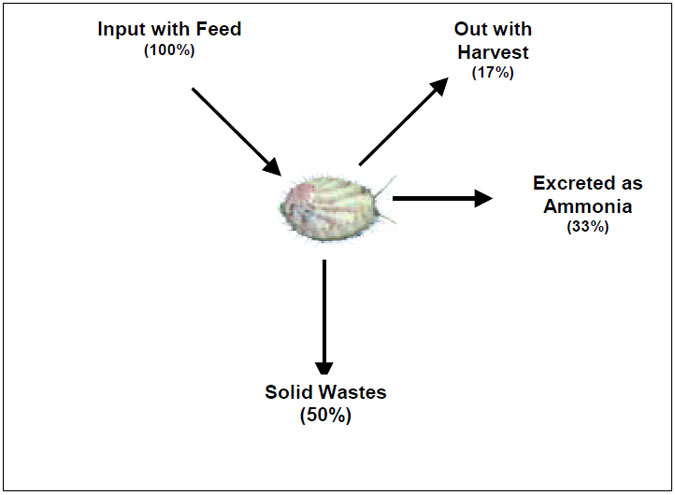
Figure 3.2: Nitrogen budget for abalone culture (Maguire, 1998).
Potential impacts are summarised in Table 3.2. A conservative hazard assessment was carried out due to the lack of scientific data on the actual impacts of offshore abalone aquaculture.
Localised impacts on water and sediment quality are possible if waste feed and faeces accumulate beneath the culture unit. However, abalone require extremely good water quality for growth and survival, and it is likely that any accumulation in waste and subsequent deterioration of water quality would impact on the cultured stock (through decreased oxygen concentrations) before serious impacts on water or sediment quality became evident.
It is possible that the use of hatchery-reared stock could compromise the genetic integrity of wild stock should there be a large-scale escape. The potential for the spread of disease/ pathogens from cultured to wild stock may also exist, however husbandry practices, including quarantine and translocation protocols, would reduce the risks involved. The collection of broodstock from the wild may potentially result in social conflict with local fishermen, although such arrangements would be subject to the agreed terms and conditions of The Abalone Management Plan.
Additional impacts from abalone ranching may include those associated with artificial habitat construction, including the consequent settlement of introduced organisms, and scouring of sediments surrounding such structures. Any such abalone ranching activities would be restricted to designated aquaculture zones, as is the case for all marine aquaculture in Crown waters, and thus related impacts would be expected to be contained within these zones.
Table 3.2: Potential environmental impacts of off-shore abalone culture.
| Resource | Source | Impact | Potential consequences | Hazard Assessment |
|---|---|---|---|---|
| Sediments | Uneaten feed, pelleted or fresh seaweed. | Accumulation beneath culture sites | Localised deterioration in environmental quality | 5 (low) |
| Cage Infrastructure | Scouring of adjacent sediments | Alteration of sediment quality. | 7 (significant) | |
| Water column | Uneaten feed | Possible release of nutrients to water column. | Deterioration in water quality | 5 (low) |
| Biological | Stock | Escape of hatchery reared stock | Possible impacts on biodiversity. | 6 (moderate) |
| Stock | Disease transfer to wild stock, genetic "pollution" | Possible impacts on wild population | 5 (low) | |
| Coastal Resources | Broodstock collection | Competition with fishermen | Social conflict | 4 (low) |
3.2 Finfish culture
Documented environmental issues related to intensive finfish cage culture are summarised in Table 3.3. Waste loadings to the environment from finfish aquaculture are directly related to farm management practices and are largely a result of on-farm feeding and fish husbandry practices. Waste loadings vary with fish species and intensity of production, which precludes the inclusion of mass-balance models showing nutrient flows in this instance. The impacts of the farm wastes on the environment are related to the geographic location of the farm and the capacity of the local environment to disperse or otherwise assimilate the wastes. Environmental hazards associated with finfish culture are site specific, however, a generic hazard assessment was conducted to identify the most important issues. The results of the hazard assessment indicated that impacts on sediment and water quality were the most important issues related to finfish culture.
Impacts on sediment quality. Sediment quality may be impacted by the deposition of solid wastes from fish cages. Solid wastes from intensive finfish culture include uneaten feed particles, fish faecal material, and smaller amounts of other detritus (such as fouling organisms) (NCC, 1989). The quantity of solid wastes discharged from a cage farm can vary significantly and will depend on: feed type (pelleted or trash fish); feeding method (hand, automatic); feed quantity and quality (as represented by the FCR) and stocking density (usually species dependant).
The solid wastes fall through the water column and settle below or adjacent to the cages. Solid wastes from fish cages can cause physico-chemical and biological changes to the sediments if they accumulate (see 6.1.1 for details). Physico-chemical changes include alteration to the sediment structure and nutrient cycling and biological changes include alterations in the macrobenthic community at the site. Impacts on sediment quality are largely dependent on the degree to which the solid wastes accumulate beneath the cages, which in turn is related to the type of waste and the bathymetry and hydrography of the site (impacts on sediments are directly related to the areal load of wastes). There are numerous studies, both overseas and in Australia, which have documented the impact of fish cages on sediments (Weston, 1986; Brown et al 1987; Gowen and Bradbury, 1987; Ritz et al. 1989). A feature of these studies is that the physicochemical impacts of cage wastes are usually restricted to within 50m of the cage unit (NCC, 1989) and that impacts on sediment quality are reversible once the source of organic enrichment has been removed. The findings of these "older" studies have recently been confirmed by a 2- year Canadian study which aimed to develop performance-based waste management standards for marine aquaculture (Brooks, 2001). Mathematical models have been developed as a management tool to predict the spread of wastes, given known production and hydrographic criteria (e.g. Cromey et al. 2001a; Cromey et al. 2001b; for review see Henderson et al. 2001).
Impacts on water quality. Soluble wastes from intensive finfish culture consist of materials excreted in urine or across the gills, and leached from the solid fraction (primarily waste feed and faeces) as it sinks. Changes to the water column will result from the soluble nutrients released, which in turn can cause alterations in the abundance and diversity of phytoplankton populations, potentially resulting in algal blooms. Other potential water column impacts include increased levels of ammonia and dissolved phosphorus at the cages. Dissolved oxygen levels can be lowered due to direct consumption by fish and also the microbial decomposition of wastes (NCC, 1989).
Table 3.3: Environmental impacts of finfish culture (adapted from FAO/NACA, 1995 and NCC, 1989)
| Resource | Source | Impact | Consequences | Hazard Assessment |
|---|---|---|---|---|
| Sediments | Discharge of uneaten feed and fish faeces. |
Accumulation of solid wastes beneath the cages |
Localised dissolved oxygen depletion. |
7 (significant) |
| Alteration of sediment nutrient cycle potentially leading to liberation of toxic gases. |
6 (moderate) | |||
| Alteration of benthic microbial and invertebrate community. |
8 (high) | |||
| Alteration of physical characteristics of the sediments. |
6 (moderate) | |||
| Discharge of chemical treatments (if applicable) |
Variable depending on type and concentration used |
Possible accumulation in sediments. |
7 (significant) | |
| Water Quality |
Discharge of uneaten feed and fish faeces. |
Leaching of nutrients into water column |
Hypernutrification leading to eutrophication. |
8 (high) |
| Localised dissolved oxygen depletion. |
6 (moderate) | |||
| Stocked fish | Excretory products | Localised increase in ammonia levels. |
6 (moderate) | |
| Chemical and drug use (if applicable) |
Variable depending on type and concentration used |
Released to the environment with unknown consequences. |
5 (low) | |
| Biological | Fish | Use of wild juvenile fish |
Decline in wild stocks leading to reduced natural productivity and loss of bio-diversity. |
7 (significant) |
| Escaped fish | Interactions with wild populations. |
5 (low) | ||
| Disease transfer | Possible transfer to wild populations |
6 (moderate) | ||
| Predator control measures |
Entanglement of native fauna. |
Reduction in biodiveristy. | 6 (moderate) | |
| Cage Infrastructure | Obstruction of native fauna |
Potential impacts on whales and dolphins. |
6 (moderate) | |
| Coastal Zone |
Obstruction of navigation |
Social conflicts | 3 (low) | |
| Conflicts over access to fishing ground |
3 (low) | |||
| Amenity value |
Culture infrastructure | Untidy sites | Visual amenity | 5 (low) |
| Servicing sites | Noise | Loss of amenity | 6 (moderate) |
Biological impacts. In addition to potential impacts on plankton and benthic communities caused by solid and soluble wastes, impacts can also occur on wild fish populations and other fauna which live adjacent to the culture unit. Wild fish can be impacted if wild seedstock are used in the cages (e.g. the southern bluefin tuna industry) as native populations can be fished beyond sustainable levels. In addition, there are genetic risks associated with hatchery-reared stock interacting with native fish populations, although most commercial salmonids are now sterile strains which cannot breed in the wild. The transfer of disease from caged aquaculture species to wild fish populations was raised as a potential problem with the Scottish salmonid industry in the 1980's and 1990's. However, researchers concluded that there was little evidence that fish farming impacts on the health of wild fish populations. Scientific evidence from Europe suggests that disease organisms that are present in farmed stocks are also frequently present in wild stocks, but farmed fish are more susceptible to clinical outbreaks of disease because of the fish farm environment. Disease outbreaks are more common in farming operations than the wild as a result of higher levels of stress, high stocking densities and more rapid transmission of disease organisms (NCC, 1990). The potential for disease transfer from wild to captive stocks is particularly high where trash fish is used as the main feed component.
Predator netting can also impact on native fauna, such as birds and seals and there also may be negative interactions between farmers and these predators. The potential obstruction and entanglement of native fauna such whales and dolphins during migration is a potential issue and should be considered in the selection of sites for finfish aquaculture.
Coastal zone. Social conflicts could arise in the coastal zone if the cage infrastructure is badly sited and interferes with existing navigation channels. Conflicts with recreational fishers and boaters could also arise if cage infrastructure is placed in lucrative fishing grounds.
Amenity value. The amenity value of the coastal zone can be affected if there is excessive noise from culture operations or the cage design is unsightly and untidy or otherwise impacts on the visual amenity.
4 Environmental Monitoring Techniques
This section discusses the broad environmental monitoring tools and techniques considered relevant to marine aquaculture in Victoria. Specific sampling methods and regimes for site characterisation, baseline and monitoring studies will be detailed in subsequent documents developed specifically for the management planning process.
The hazard assessment identified that the most important environmental issues related to bivalve culture were impacts on sediment quality. Similarly, the most relevant issues for finfish culture were impacts on water and sediment quality. Consequently, this section will investigate appropriate methods of monitoring the impacts of aquaculture on water and sediment quality. Other environmental issues identified in the previous section will be dealt with in the management plans for each aquaculture area and, where necessary, dealt with as part of the licence conditions (see section 6).
Appropriate techniques for monitoring the environmental impacts of aquaculture operations have been intensively studied over the past two decades and are summarised in Table 4.1. The GESAMP Expert Working Group on Environmental Impacts of Coastal Aquaculture conducted a review of appropriate monitoring for coastal aquaculture under the auspices of UNEP. The working group suggested that monitoring effort should be related to the scale of impact of an aquaculture operation (GESAMP, 1996). The scale of impact of an aquaculture operation is a function of the scale and intensity of the operation and the sensitivity of the proposed location. The rationale behind the parameters selected for monitoring impacts on water and sediment quality is discussed below.
4.1 Sediment Quality
4.1.1 What Are We Looking For?
- Organic Enrichment. In monitoring the impacts of both finfish and shellfish farms on marine sediment quality we are looking for evidence of accumulated organic material beneath the aquaculture operation. Similar to other sources of organic materials this can cause both physico-chemical and biological alterations in the sediments.
-
- Physico-chemical changes. The solids from finfish farms are enriched with carbon, nitrogen and phosphorus relative to the natural sediments, and the characteristics of the sediments may be altered below or adjacent to the farm. Solids from shellfish farms are generally enriched with carbon and nitrogen but not phosphorus (Kautsky and Evans, 1987). The input of carbon to the sediments will initially mean that the productivity of the benthic community will increase, with an associated increase in oxygen consumption by that community. However, if organic matter accumulates to the extent that the rate of oxygen consumption exceeds the rate of supply, then anoxic conditions may develop at the sediment-water interface. This can have potentially serious consequences for both the fish farmer and the local marine environment and lead to the liberation of gases such as ammonia, methane and hydrogen sulphide from the sediments.
Table 4.1: Variables often used to monitor the ecological effects of coastal aquaculture wastes for the purposes of environmental protection (GESAMP, 1996). H=High, M=Moderate, L=Low.
| Variable | Usage | Cost | Value | Comments |
|---|---|---|---|---|
| Sediment Chemistry |
||||
| Redox potential (Eh) | M | L | H | Measurement is rapid and low cost beyond initial equipment purchase, thus permitting extensive spatial surveys. |
| Depth of visual Eh discontinuity (redox) |
L | L | L | Only semi-quantitative and sometimes difficult to determine |
| Total Organic Carbon (TOC) |
H | M | M | Most easily measured by elemental analyser, but can also be done by wet chemistry. |
| TKN/TN | L | M | M | Provides similar information to TOC. TN data are provided simultaneously with TOC if elemental analyser used. |
| Benthic Biota | ||||
| Visual presence of Beggiatoa |
H | M | L | Beggiatoa is a filamentous bacteria that forms a white mat on the sediment surface in areas of intense organic enrichment. A highly reliable and easily observed indicator. Its presence suggests substantial disruption of natural benthic processes. |
| Macrofaunal community structure |
H | H | H | Expensive and requires taxonomic expertise that may not be widely available, but provides evidence of benthic impact. |
| Visual survey of large invertebrates and demersal fishes |
H | L | L | Documentation by still photos or videotape. Observations typically only qualitative. |
| Water Chemistry | ||||
| Dissolved oxygen | H | L | H | Measurement by wet chemistry or probe. |
| Biochemical oxygen demand (BOD) |
H | L | H | Widely used for measurement of effluent quality for land-based systems. |
| Suspended solids (SS) |
H | L | M | Widely used for measurement of effluent quality for land-based systems. |
| PH | L | L | L | Useful as a measure of impact only in some forms of pond culture, but often measured as an ancillary variable. |
| Transparency (Secchi disc / NTU) |
H | M | M | Non-specific indicator of questionable value in many locations. |
| Dissolved inorganic nutrients |
L | M | M | Particularly appropriate when the ecosystem is N or P limited |
| Phytoplankton Biomass |
||||
| Chlorophyll | L | M | H | Useful as an index of eutrophication, but unlikely to be of value on an individual farm basis. |
In Port Phillip Bay, sedimentation of the substrate may prevent the aerobic nitrification process occurring, potentially perpetuating the recycling of ammonia. On a large scale, or in a confined area, this may result in eutrophication. On the scale of marine aquaculture in Victorian waters however, any such impacts on the sediment, should they occur, would be localised to within the aquaculture zone. Such processes would need to occur bay-wide for the risk of eutrophication to occur (Harris et al. 1996).
- Biological changes. Increased sedimentation from finfish and shellfish aquaculture can effect changes in the macrobenthos within the sediments by physically smothering the sediment or making conditions unsuitable for a diverse community of invertebrates. In extreme situations where anoxic conditions have developed there could be a complete loss of the macrobenthic community. in situations where this has been found beneath finfish aquaculture areas it is usually restricted to directly beneath the cages (NCC, 1989). Under normal circumstances, however, there is a gradual change in community structure in areas affected by organic enrichment with increasing dominance of fewer tolerant species, such as polychaetes. This is known as the opportunistic zone. Where there is light organic enrichment, such as occurs beneath shellfish aquaculture areas, the existing benthic community becomes more productive and gradually returns to background levels.
More specifically, studies have shown that benthic communities will return to normal after the source of organic enrichment has been removed (Pearson and Rosenberg, 1978). This has also been shown to be the case in Australia (Ritz et al, 1989). After a 12 month fallowing period the cage site had returned to aerobic conditions although levels of organic matter were still elevated compared with control levels (McGhie et al. 2000).
NCC, (1989) summarised the effects of finfish aquaculture on benthic infauna as follows: - Anoxic zone: If present usually restricted to the sediments directly below the cages.
- Opportunistic zone: Restricted to immediate vicinity of the cages or up to 30m from the site.
- Return to background: Normally occurs within 30m of the farm.
- Debris. The physical structure of sediments can be influenced by a covering of shells or other debris from the aquaculture site. The deposition of live mussels, shell materials and other detritus from aquaculture operations can provide sites for attachment of invertebrates.
4.1.2 How can we monitor change?
- Photographic/Video Monitoring. This can be performed through underwater video surveillance or photographs carried out by divers at both shellfish and finfish farms. The length, number and position of transects to be videoed or photographed will be site specific and depend on the monitoring strategy that is adopted. For inter-tidal shellfish farms photographs of the lease area at low tide are normally sufficient. Compared with other forms of monitoring (chemical and biological analysis), this is relatively inexpensive and readily provides qualitative information on sediment quality. This form of monitoring will identify areas of organic enrichment (e.g. Beggiatoa bacteria, changes in sediment colour) as well as inorganic debris beneath the aquaculture zone. It can also be used to monitor changes in the character of the lease area over time. In finfish culture, video surveys can show the spread of fish feed from the cages and visual changes in sediment condition.
- Redox Potential. Redox potential is a standard method for categorising the physio-chemical condition of marine sediments in many parts of the world. It is generally regarded as a reliable guide to the amount of oxygen in the sediments. A measure of redox potential of sediment cores enables reliable comparisons to be made of the intensity of reducing conditions in sediments from place to place (Whitfield, 1969). This serves as a guide to the biological condition of the sediment and the degree of organic loading to which it is subjected (Pearson and Stanley, 1979). Studies undertaken in Tasmania have correlated the relationship between sediment structure, redox levels and changes in community structure (MacLeod, 1996). In Port Phillip Bay studies have indicated that dissolved oxygen is lost within the top 3mm of the sediment (Burke 1995) which may limit its use for the purposes of general environmental monitoring. However, redox may detect changes in Eh in aquaculture situations where an impact is occurring eg. beneath cages or longlines, and therefore should be retained as an analytical tool for environmental monitoring in Victorian marine aquaculture.
- Visual Assessment of Sediment Quality. Visual assessment of sediment chemistry can be carried out in conjunction with redox analysis, and is usually a written description of the core samples prior to redox measurement. The depth of the core and changes in sediment composition and colour with depth can give valuable information on the degree of organic enrichment.
- Sediment Grain Size. Grain size gives an indication of changes in sediment condition beneath the aquaculture area compared with control sites. If used as part of a baseline study it provides useful data on the sediment and flushing characteristics of the area.
- Carbon/Nitrogen Ratio. The C/N ratio gives a direct measure of the degree of organic enrichment compared with baseline or control levels. A variation of this method is stable isotope analysis where the source of organic enrichment can be pinpointed.
- Benthic Community Structure. Benthic community structure is an important measure of the ecological effects of aquaculture. Changes in community structure of the benthos between aquaculture and reference sites may be elucidated through benthic sampling. In Tasmania a method has been developed to evaluate the health of the macrobenthic community under fish cages (Ritz et al, 1989). The ABC (abundance biomass comparison) method relies on the relationship between changes in species diversity and population numbers, and the degree of disturbance of the environment. This method enables the collection of a relatively small number of samples to be used to judge whether a site is considered undisturbed, moderately disturbed or grossly disturbed (DPIWE, 1999). It is particularly useful in estimating the health of sediments at sites where there is no existing background data, and can also be used as part of a baseline study for new farms.
4.2 Water Quality
4.2.1 What are we looking for?
As previously outlined, changes in water quality may occur through respiration of the stock, breakdown of aquatic wastes in the water column and sediments, alteration of benthic communities, and indirectly through impacts on phytoplankton populations. In routine monitoring we are looking for changes in water quality which may be detrimental to aquatic life, both internally in the farm and externally in the wider environment. Monitoring should also be undertaken to ensure that any environmental quality objectives for the area are maintained (e.g. as laid out in the State Environmental Protection Policy). In shellfish culture areas, regular monitoring is required to ensure that water quality will not adversely affect product quality, although this is an impact of the environment on aquaculture rather than vice versa.
4.2.2 How can we monitor change?
- Dissolved Oxygen. Dissolved oxygen is a critical parameter for aquatic life and adequate levels must be maintained to ensure good growth and survival of aquaculture species. Dissolved oxygen levels fluctuate naturally in the marine environment due to diurnal variation in phytoplankton photosynthesis and respiration. After this has been taken into account, however, a marked reduction of oxygen levels at the aquaculture unit compared with a control site could indicate overstocking, and/or organic waste accumulation. Monitoring of dissolved oxygen levels is a useful tool for aquaculturists who can utilise the data for stock/farm management purposes.
- Transparency. Water transparency can be measured using a secchi disk, and over a period of time gives a useful indication of suspended solids or algal biomass in the water column. Secchi disk measurements do not differentiate between different causes of water turbidity.
- Dissolved inorganic nutrients. Dissolved inorganic nutrients are released from the stock in the form of excreta and also leached from sedimenting wastes or the sediments. Elevated concentrations of some dissolved nutrients (e.g ammonia) can be harmful to aquatic life and can be indicative of overstocking or waste accumulation.
- Phytoplankton biomass. Phytoplankton species diversity and abundance should be monitored near to shellfish farms to detect any algae which are toxic or which can cause disease in humans. This is currently carried out under the VSQAP monitoring program.
- Bacteria. Background monitoring of the bacterial quality of shellfish culture areas is important to detect any potential threats to shellfish quality. This is currently carried out under the VSQAP monitoring program.
- Other Pollutants. Other potential pollutants such as heavy metals, therapeutics and pesticides should be monitored periodically in the product, particularly in shellfish growing areas. This is currently carried out under the VSQAP monitoring program.
5 Regulation of the Marine Aquaculture Industry
5.1 Interstate
Approaches to the regulation of the marine aquaculture industry interstate provides useful insight into factors which should be taken into account when developing a management framework for Victorian marine aquaculture. This review focuses on Tasmania and South Australia given their relatively close geographic proximity and similar climatic characteristics to Victoria.
Marine aquaculture in Tasmania can only take place in State waters where a Marine Farming Development Plan is in force. These development plans are prepared at a regional level and include an environmental impact assessment of the region and recommend zones suitable for marine aquaculture. There are currently nine development plans with a further two being formalised. The development plans stipulate general management controls, which are measures that are necessary to manage and mitigate negative effects from aquaculture development. These management controls are then incorporated into individual aquaculture licences. When the plans are formalised, tenders are invited for the leases identified within the plan.
A similar approach is taken in South Australia, where regional Aquaculture Management Plans are prepared to identify areas of importance which require some form of protection or buffer, and which are to be excluded from aquaculture development. Other areas, called "Aquaculture Zones" may be made available for aquaculture development. Aquaculture SA is currently developing a framework for managing the sustainable development of aquaculture. All new aquaculture licences issued by PIRSA from 2001 onwards will involve assessing the proposed aquaculture in terms of it's ecological sustainability. Licence conditions will be applied so that the Ecologically Sustainable Development (ESD) targets of the site can be effectively measured. Licences will be issued for 12 months and can be revoked at any time if the licence conditions are not met. In addition, a de-merit system will operate where points can be added to the licence for failure to comply with consent conditions.
Shellfish Quality Assurance Protocols. As outlined in Section 5, shellfish aquaculture is particularly susceptible to the external environment and there are Federal guidelines developed to assist the states in assuring that the areas designated for shellfish culture are environmentally suitable. ASSCP (2000) identified five types of hazard which could be detrimental to shellfish aquaculture areas:
- Enteric bacterial pathogens (e.g. Salmonella sp.)
- Enteric viral pathogens (e.g. Hepatitis A)
- Biotoxins (from phytoplankton)
- Chemical contaminants (e.g. heavy metals and pesticides)
- Naturally occurring bacterial pathogens.
Protocols for the monitoring of these hazards have already been laid out (ASSCP, 2000) and the results can be used to classify shellfish growing areas into three primary classifications:
- areas where the shellfish may be sold directly for human consumption;
- areas where the shellfish have to be depurated; and
- areas in which shellfish harvesting is prohibited.
Each state conducts periodic monitoring of pathogens, phytoplankton and pollutants to safeguard bivalve aquaculture areas.
5.1.1 Shellfish aquaculture regulation
Interstate approaches to the regulation of the impacts of shellfish aquaculture on the environment are summarised below. Due to the lower risk of serious environmental impact associated with shellfish aquaculture management controls and monitoring protocols are generally less intense.
Tasmania. In Tasmania, management controls on aquaculture licences stipulate that there must be no unacceptable environmental impact outside the boundary of the lease area. Relevant parameters must also be monitored in accordance with the requirements of the licence. Licence holders are required to carry out baseline studies (or Initial Monitoring Studies) which include bathymetric studies, habitat profiles and seabed characteristics, video, sediment chemistry and benthic monitoring. Baseline studies are also required for lease areas undergoing greater than 10% expansion or being relocated. Existing farms have to conduct initial monitoring surveys which vary depending on the production of the farm. High production farms (>100,000 dozen or 100 tonnes) must undertake a full baseline survey, plus provide current data. Low production farms must only provide current data, bathymetric profiles and habitat and seabed characteristics. Protocols have been developed to standardise methods of conducting baseline studies. In addition, DPIWE licence controls are exercised in relation to:
- Environmental carrying capacity – limits are placed on length of longline per ha of lease area (1.0-1.1km/ha) and all longlines are to be kept at least 1 m clear of the seabed. Stocked racks are limited to 1km per ha of lease area or 3km of post and wire equipment.
- Environmental monitoring – programs have been specified for inter-tidal and sub-tidal culture. Baseline studies are required for new lease areas and expansions greater than 10%. The numbers and biomass of shellfish held in a lease area must be reported annually.
- Introduced species. Introduced species such as Pacific oysters must be 100% sterile.
- Seagrass. Seagrass biomass must be assessed by the lessee annually.
- Waste – wastes from harvesting and processing must be disposed of in a manner which does not affect the ecology of the marine environment.
- Disease – protocols for disease notification and surveillance.
- Visual impacts – conditions are imposed on colour, size and shape of buoys and netting and other floating equipment and other factors influencing visual amenity.
- Access. Marking of external boundaries of lease area.
- Other controls relating to noise, debris, predator control etc.
Annual monitoring is not required at present, but the situation is currently under review.
South Australia. In South Australia, shellfish culture is essentially regulated through licence conditions such as stocking densities or length of culture infrastructure. Mussel stocking densities are restricted to between 500,000 at 100 mm in size, and 30,000,000 at 3 mm. Oysters are restricted to a maximum of 3km longline or 1km racking per unit and there must be 5m between units. Applications from prospective shellfish farmers are submitted to PIRSA along with information on:
- the physical and biological characteristics of the site (exposure, tides, water movement, water quality, sources of pollution etc);
- Farm operation (species, culture method, tonnage etc);
- Water quality factors (cleaning, chemical use, feeding, fallowing, processing, expected impacts);
- Site activity (access, noise, vessels);
- Other resource issues in the area;
In addition a baseline survey must be completed with at least one video transect passing through the centre of the site. Sediment samples are collected at the start, middle and end of the transect and analysed for particle size and organic matter. A map is also required showing the distribution of substrate types as well as the location of dominant flora and fauna on the site.
When the application is received by PIRSA, it is assessed on the basis of Ecologically Sustainable Development. Licence conditions are then drawn up to address the specific impacts or risks identified in the assessment process and are essentially site specific.
Monitoring and reporting is required once per year and involves a video transect through and outside the lease, and sediment samples from inside the site.
5.1.2 Finfish culture regulation
Due to the higher risks associated with finfish aquaculture, both Tasmania and South Australia require more detailed baseline studies and regular monitoring.
Tasmania. In Tasmania, marine cage culture is dominated by salmonid aquaculture. An applicant for a marine finfish lease area in Tasmania must undertake a detailed baseline study to evaluate the proposed site. Baseline studies are also required for lease areas undergoing greater than 10% expansion or being relocated. This baseline survey must conform to pre-determined protocols relating to current measurement, bathymetry, seabed characteristics/ habitat type, underwater video survey, sediment chemistry and biological analysis.
Similarly to shellfish leases, the conditions of finfish licences dictate that there must be no unacceptable environmental impact outside the marine farming lease area. Conditions are also specified relating to:
- Carrying capacity
-
- Maximum stocking density (25 kg/m3)
- Fallowing of cage sites if bubbles of hydrogen sulphide and methane are observed.
- Nets must be at least 1m clear of the seabed at low tide.
- Monitoring and Reporting
-
- Annual records on fish feed usage;
- List of names and quantities of chemicals used on site;
- Location, size and stocking rates of cages and fallow areas;
- Environmental monitoring and reporting of results required.
- Waste. Wastes from harvesting, processing and fouling organisms must be disposed of in an acceptable manner.
- Disease. Diseases must be notified to the appropriate authorities.
- Visual controls. Standards related to farming structures and equipment.
- Access. External boundaries of the lease must be marked.
- Other. Conditions are stipulated with respect to excess noise, maintenance of equipment and predators.
South Australia. An applicant for a marine cage culture development in South Australia is currently assessed against the relevant development plan. Development applications requiring the propagation or rearing of finfish in marine waters are also referred to the Environmental Protection Authority (PIRSA, 2000). Where the application affects the coastline or coastal seabed it is also referred to the Coast Protection Board. The application is advertised for public comment.
Under the existing regulatory system, a prospective aquaculture operator requires a licence to farm fish and may also require a licence under the Environmental Protection Act (1982). The information required is similar to that required for shellfish culture (including a baseline study) and the assessment process also is carried out with social, economic and ecological impacts in mind. The fish farming licence contains provisions for managing both environmental impacts and operational activities associated with aquaculture. These provisions include a requirement to undertake environmental monitoring and include conditions to manage environmental impacts, such as the periodic fallowing of aquaculture sites. The monitoring program includes details of farm management at the lease area (location, number and size of all structures; stocking densities and species; stocking and harvest numbers; and disease outbreaks). Monitoring includes benthic sediment analysis (redox potential and benthic communities) at pre-determined sample sites.Licence conditions for tuna farms in South Australia incorporate the following conditions
- Marked off areas. This defines the lease area.
- Permitted species and use. Only species on the licence can be grown on site and the lease can only be used for the permitted use.
- Permitted methods. Stipulates farming methods to be used with regard to:
-
- Stocking rate (number, weight, value of each species/ lifestage)
- Chemical usage
- Number of cages and layout
- Environmental monitoring program.
- Management strategy with regard to interactions with seabirds and marine mammals.
- Fish brought from interstate
- Disposal of water and packaging used to transport fish
- Methods of disposal of diseased and dead fish.
- Location of seacages, marking and maintaining the site.
- Site inspection and supervision.
5.2 Overseas
The European Union has funded a two year MARAQUA Concerted Action program to establish scientific guidelines for Best Environmental Practice for the regulation and monitoring of marine aquaculture throughout the EU (Fernandes et al 2000). This project found that despite the different approaches, species, methodologies used and geographical locations, strategies to address environmental issues related to marine aquaculture were remarkably similar in Europe and North America (Read et al 2001).
5.2.1 Shellfish culture
Approaches to the regulation of shellfish aquaculture in major producing countries in Europe (Table 5.1) and New Zealand are summarised here. In general, most countries adopt a blend of self-monitoring and authority monitoring to regulate the areas in which shellfish are grown and the quality of the product. The emphasis is usually on protecting aquaculture from the environment rather than vice versa. The EU has issued directives on the quality of water required for shellfish culture and for product safety.
Table 5.1: Regulation and monitoring of shellfish aquaculture in selected European countries (adapted from Fernandes et al 2000).
| France | Spain | ||
|---|---|---|---|
| Regulation | |||
| Environmental standards | EU standards | Water quality for shellfish culture | |
| Food standards | Bacteria and algal toxins | EC directives: maximum residues. | |
| Medicines/ pesticides | Licence required | Controlled by vets | |
| Environmental monitoring | |||
| Self monitoring | - | - | |
| Authority monitoring | Water quality for shellfish – algal toxins and phytoplankton monitored twice per month. | Periodic water quality monitoring. Weekly red tide monitoring. | |
| Food Safety | |||
| Food Safety | Bacteria/ phytoplankton monitoring in flesh prior to marketing | - | |
| Authority monitoring | Bacteria, phytoplankton toxins in shellfish flesh (twice monthly) | Compliance with food standards monitored yearly. | |
France (after Dosat and de la Pomelie 2000)
Marine aquaculture production in France is dominated by the cupped oyster (Crassostrea gigas), with an annual production of 150,000 tonnes. Shellfish farmers in France must obtain a permit to set up an aquaculture site in marine waters which allows the holder to use maritime public property. The permit lists the species which may be farmed, the level of production, the location, and some general specifications of the operation. Some additional conditions may also be added regarding monitoring requirements.
In France, areas for shellfish are zoned to protect the consumer from bio-accumulation of heavy metals and bacterial toxins. Shellfish from each zone are monitored twice per month for microbiological parameters and once per year for heavy metals. There is no monitoring of the impact of shellfish culture on the environment.
Spain (after Sanchez-Mata and Mora 2000).
In Spain, aquaculture production is dominated by the extensive culture of shellfish, particularly mussels. Production was around 260,000 tonnes in 1998. To support each application for a shellfish aquaculture licence, the following documentation is required:
- Baseline study of physico-chemical and biological characteristics of the area. Includes also management strategies for depuration, chemical usage, predators and disease monitoring.
- Socio-economic study of the area.
- Technical study addressing visual impact and noise issues.
- Construction plans.
- Photographs of the area and alternative sites
- Financial accounts
- Assessment of the impact of the development on water and sediment quality.
Environmental monitoring for shellfish aquaculture in Spain is the responsibility of regional environmental agencies and concentrates on red tide monitoring which is carried out on a weekly basis. Periodic monitoring of other water quality parameters is also carried out.
New Zealand.
New Zealand operates a dual consent process for controlling the development of marine aquaculture. Applicants must first obtain a Resource Consent which allows them to utilise natural resources for the aquaculture in areas approved under the district plan. They must then obtain a permit under the Fisheries Act to culture aquatic species.
The Resource Consent is the main instrument for controlling the environmental effects of aquaculture as applicants have to submit a general environmental assessment report including data on sediment characteristics and condition and important habitats. This information is used to assess the sustainability of the proposed development. The Fisheries Permit application process considers the impact of the development on other users such as: custodial users; commercial and recreational fishermen; and other aquaculturists. In both applications, the onus is very much on the applicant to demonstrate that the effects of the development will not be detrimental to the environment or other users.
New Zealand is currently reforming it's aquaculture consent process to streamline applications into a one-stop shop for applicants. In New Zealand there is no requirement for on-going monitoring, although the Authorities could make that a condition of the licence, if required. Regular monitoring of water quality in shellfish growing areas is carried out for export certification purposes, this is fully funded by industry.
5.2.2 Finfish culture
The three European countries selected (Table 5.2) have adopted different approaches to regulation and management of the finfish culture industry. In all three countries, the predominant culture species is salmonids with Denmark producing 8,500 tonnes in 1998, Norway producing 460,000 tonnes and Scotland producing 112,000 tonnes.
Denmark (after Pederson 2000)
Before establishing or extending a finfish cage farm in Denmark a permit must be obtained which contains the following restrictions:
- Maximum yearly output of total nitrogen and phosphorus;
- Feed type and composition of feed;
- Maximum yearly allowance of feed (calculated on basis of FCR 1.5:1);
- FCR must not exceed 1.6:1
- Killing protocols
- Environmental monitoring requirements (sediment nutrient analysis 2x year).
- No active removal of sediments
- Records must be kept and sent to regulatory authority each year.
In Denmark, instead of the carrying capacity for an individual site being assessed, an overall limit on nutrients and organic matter into marine waters has been set. Within this allocation, marine aquaculture has been allowed a quota of 550 t nitrogen and 54 t phosphorus. The impact of the farm is not assessed in relation to the site in which it is established, but on a simple mass balance between nutrients input with feed and harvested as fish.
Table 5.2: Regulation and monitoring of finfish aquaculture in selected European countries (after Fernandes et al 2000).
| Denmark | Norway | Scotland | |
|---|---|---|---|
| Regulation | |||
| Carrying capacity | Maximum waste loading of 550 tonnes N and 54 tonnes P | Maximum density 25 kg/m3. | Maximum weight of fish on site. |
| Environmental standards | Nutrient output, feed type, FCR. | Nutrients, organic matter, dissolved oxygen, sediment quality (benthos). | Hydrological characteristics, biological, sediment and water quality |
| Hydrological characteristics, biological, sediment and water quality | - | - | Maximum residue limits for chemicals. |
| Medicines/ pesticides | Vet prescription/ not permitted | Permission required | Permission required |
| Environmental monitoring | |||
| Self monitoring | Water quality 12 x year. Sediment quality 2 x year | - | Near-field sampling following an agreed program. |
| Authority monitoring | Compliance with finfish standards | 15% of fish farms monitored every year using the MOM system on sediments | Audit self-monitoring. Consent compliance for medicines, Environmental impact. |
| Food Safety | |||
| Authority monitoring | Periodic compliance with standards | 10% finfish checked every year for chemical residues. All medicated fish checked. | Trace compounds in fish tissue. |
In Denmark, monitoring to assess compliance with licence conditions is the responsibility of the fish farmer who also bears the cost. Authorised companies collect and analyse samples and report the results to the relevant authority. The authority can carry out additional sampling, if required.
Norway (Maroni, 2000)
In Norway, the marine aquaculture industry is strictly regulated with the system based on environmental standards designed to meet the requirements of all users of the marine environment. When applying for a licence, a fish farmer must supply data on: farm location; current measurements; sediment quality; bathymetry; and planned production.
The conditions on the licence issued include:
- Maximum production volume 12,000 m3
- Defined protocols for use of medicines and disinfectants
- Obligation to send detailed annual report to the authorities - records must be kept at licence level (handling and delivery of dead fish, feed purchases, anti-fouling chemicals); site level (fish health, lice, use of medicines and chemicals, escapes); and cage unit level (stocking, density, net depth, feed consumption, escapes, harvested/mortalities).
Coastal waters and fjords in Norway have been classified according to their environmental quality and sensitivity to pollution. Each licence application is assessed to the "total carrying capacity" of the whole area. Fish farms in Norway are increasingly being monitored by the use of MOM (Modelling-Ongrowing-Monitoring) systems which is based on integrating elements of the EIA, monitoring of impacts and environmental quality standards into one system (Evrick et al. 1997; Hansen et al. 2001). The amount of monitoring which has to be carried out is dependent on the level of environmental impact, with the monitoring carried out on the sediments under farms. There are three types of monitoring survey of increasing complexity and accuracy (A, B and C). The A level investigation is a simple measure of the sedimentation rate beneath the cages. The B level investigation is a simplified sediment survey which looks at trends in sediment quality. The C level investigation is a comprehensive investigation of the benthic macrofaunal community.
In Norway, it is the responsibility of the farmer to document through monitoring that the farm is not causing unreasonable effects. The authorities inspect 15% of farms each year and audit their production and monitoring records.
Scotland (Henderson and Davies, 2000)
In Scotland a developer wishing to discharge effluent from a fish farm must obtain a discharge consent from the EPA (SEPA, 1998) as well as a site lease. Applications for fish farms over 100 tonnes, with a cage surface area greater than 1,000 m2, or located in "sensitive" areas, must carry out an Environmental Impact Assessment. Applicants must provide the EPA with the following information:
- Plan showing proposed cage locations within lease area;
- Bathymetric maps;
- Details of cage design;
- Details of fish food, N & P content and feeding method to be used;
- Documentation on the use of medicines and chemicals;
- Hydrographic survey data
- Pre-development or ambient seabed characteristics and benthic biological survey data.
The application is assessed with reference to the acceptability of the site for aquaculture, which is a function of its current environmental usage (conservation status, other users) and environmental status (sediment quality, water quality, biological quality and hydrography). Consent conditions are site specific and are numeric or descriptive, including: cage positions and numbers; species to be cultured as well as conditions to control environmental impact.
Carrying capacities are stipulated to achieve a balance between fish biomass and environmental impact. A consent contains a limit on the maximum permitted weight of fish (biomass) to be held at any one time to achieve sustainability, to prevent anoxic and polluted sediments and associated deleterious benthic effects occurring outside the accepted zone of influence. Deposition models (e.g. Cromey et al. 2001a) are used to assist in the decision-making process and biomass levels will reflect the capability of the site to disperse and rework organic wastes.
Monitoring programs are in place to ensure effective regulation of marine cage farms and protocols have been developed for baseline studies, operational monitoring and assessing site recovery. SEPA routinely operates a cost-effective near cage rapid impact assessment strategy for organic wastes (based on a combination of video, benthic samples and sediment chemistry), which replaces expensive transect work. Monitoring is scaled to the size of farm, hydrographic character and site sensitivity. Due to the large number of cage farms a policy of self monitoring with audit has been adopted.
6 Environmental Monitoring Strategies for Victoria
NRE Fisheries is committed to the ecologically sustainable development of the Victorian aquaculture industry through its Aquaculture Development Strategy (NRE Fisheries, 1998). This strategy commits the Victorian Government to:
- Establish a regulatory and management framework under which ecological sustainability will be maintained;
- Implement effective environmental management performance standards for aquaculture development.
6.1 Victorian Legislative Setting
The aquaculture industry is subject to a complex array of regulation, mostly at State Government level (ORR, 1999). Different authorities within the State government administer the key pieces of legislation (Table 6.1). In addition, local government is also responsible for the administration of regulation impacting on coastal aquaculture.
Department of Natural Resources and Environment. DNRE is the key Agency for regulating the aquaculture industry in Victoria, through it's administration of the Fisheries Act 1995; the Lands Act 1958; Flora and Fauna Guarantee Act 1988; and the Coastal Management Act 1995.
In addition to these pieces of legislation, DNRE is also guided in its approvals process by several policy and strategy documents, including:
- Victorian Aquaculture Strategy (see above).
- Australian/Victorian Translocation Guidelines.
- Victorian Coastal Strategy (Victorian Coastal Council, 2001). This strategy recommends the promotion of sustainable aquaculture development.
- Victoria's Bio-diversity Strategy (DNRE, 1997). Provides a framework for the conservation and management of bio-diversity.
- Port Phillip Bay Environmental Management Plan (DNRE, 2002). Provides a framework for the environmental management of Port Phillip Bay.
Environment Protection Authority (EPA). The EPA is another key player in coastal aquaculture management, particularly for land-based sites where it licences waste discharge and sets initial requirements for monitoring. The EPA only licences certain activities which are defined through regulation. At present, in the aquaculture industry, only land-based aquaculture operations with a discharge of greater than 0.2 ML/day require an EPA works approval. The legislation that dictates which activities require EPA licensing does not cover offshore aquaculture, and so the EPA has no responsibility for regulation of offshore aquaculture. The EPA considers that there are adequate means to address environmental issues related to aquaculture through the Fisheries Act 1995 and the Coastal Management Act 1995, however it can intervene if an aquaculture operation causes pollution.
Under the Environment Protection Act 1970, the EPA has developed State Environmental Protection Policies (SEPP) for Victorian coastal waters. The SEPP for Port Phillip Bay (Victorian Government Gazette No. S101. Wednesday 27 August, 1997) recognises aquaculture as a beneficial use which must be protected, in some segments of the bay. Clause 17 requires aquaculture to be managed in accordance with best practice to ensure protection of beneficial uses and ecological processes of Port Phillip Bay. In order to maintain aquatic ecosystems, aquaculture should not substantially alter ecological processes.
Table 6.1: Key Legislation Governing Coastal Aquaculture in Victoria.
| Legislation | Administration | Purpose |
|---|---|---|
| Fisheries Act 1995 | NRE Fisheries (DNRE) Minister for Agriculture and Resources. | Licensing of aquaculture activities & regulation of licensed activity. |
| Lands Act 1958 | DNRE Minister for Conservation and Land Management. | Grants leases (max 21 yrs) over unreserved Crown land for industrial, commercial, agricultural and other purposes. |
| Environment Protection Act 1970 | EPA Minister for Conservation and Land Management. | Licensing of Works Approvals and Discharge which are required before wastes can be discharged.. Application fee and annual renewal fee. |
| Flora and Fauna Guarantee Act 1988. | DNRE Minister for Conservation and Land Management. | Restrictions on threatened and non-native species. Provisions for exemptions. |
| Planning and Environment Act 1987. | Dept Infrastructure (Local Govt Planning Schemes) | Planning permits are usually required for aquaculture activities for the use and development of land at the commencement and expansion of operations. |
| Coastal Management Act 1995. | DNRE | Sec 37 requires the consent of the Minister for use and development of coastal Crown land. Developments must conform with guidelines for Coastal Siting and Design and be consistent with Coastal Action Plans and Coastal Management Plans. |
This clause also stipulates that aquaculture projects must have a provision for site rehabilitation to ensure that private operators do not pass on the costs of rehabilitation to the government and community. Similar SEPPs have been published for other coastal areas e.g. Western Port.
Department of Infrastructure (DoI). The DoI administers the Planning and Environment Act 1987 through local planning schemes. Land-based aquaculture is subject to regulation under municipal planning schemes. Offshore aquaculture is not covered by planning schemes but, depending on the scale of the operation, may still require an Environmental Effects Statement. Otherwise applications will be considered under the Coastal Management Act 1995.
6.2 Current Management Controls For Marine Aquaculture
Aquaculturists currently are required to apply for an Aquaculture Licence under the Fisheries Act, and conditions on licences for mussel farms require declaration of the following:
- Species
- Location of lease area
- Culture method
- Navigation/ identification marking, equipment maintenance.
- Spat collection
- Record keeping
- Translocation protocols.
The licensed areas are inspected annually by Fisheries Officers or if the licensee applies to expand or vary their licence. There is currently no requirement for environmental monitoring at the existing marine aquaculture areas.
NRE Fisheries currently controls the Victorian Shellfish Quality Assurance Program (VSQAP) in conjunction with industry to ensure that water quality in the shellfish growing areas will not adversely affect product quality. VSQAP is performed under the operational guidelines of the Australian Shellfish Quality Assurance Program (ASQAP). WATER ECOscience is the agency currently responsible for monitoring and reporting under VSQAP. The program was established to ensure that mussels are fit for human consumption when harvested, and to maintain Australian Shellfish Sanitary Control Program (ASSCP), and Australian Quarantine Inspection Service (AQIS) accreditation for domestic and export consumption of farmed Victorian mussels.
The monitoring program is divided into two parts, phytoplankton/biotoxin testing and microbiological testing. Routine fortnightly biotoxin sampling is undertaken where samples of phytoplankton and mussel tissue are taken from each growing area (Clifton Springs, Grassy Point, Dromana, Beaumaris and Flinders), and analysed for paralytic shellfish poison (PSP) concentrations. Amnesic shellfish poison (ASP) is routinely sampled for at Clifton Springs and Flinders only. Other growing areas are sampled for ASP only when phytoplankton concentrations indicate this is necessary. Testing for diarrhetic shellfish poison (DSP) is also undertaken when concentrations of the relevant phytoplankton indicate this is necessary. Microbiological sampling is undertaken at least six times annually at Clifton Springs and Grassy Point, and at least twelve times annually at Flinders, Dromana and Beaumaris using an adverse pollution strategy. Testing is carried out immediately a high rainfall event, or sewage spill occurs. If neither event occurs over a long time frame, such as two months, then microbiological sampling is undertaken on a routine biotoxin sampling event. The results of the microbiological testing are used to investigate the efficacy of the current rainfall triggers and protocols used to suspend and recommence harvesting operations.
6.3 Scope of Victorian Marine Aquaculture Management Plans
The recommendation of several new aquaculture areas along the Victorian coastline by the Environment Conservation Council (ECC, 2000) has hastened the need to develop management plans for these areas prior to development. The Fisheries Act 1995 stipulates that management plans must be prepared for each fishery reserve (sec. 28 (5)) as soon as possible after the fisheries reserve is declared under s.88. It also stipulates that the management plan must specify policies and strategies for the management of the fishery on an ecologically sustainable basis (sec. 29 (1)).
The purpose of management plans for Victorian marine aquaculture is different from those prepared in Tasmania and South Australia as the aquaculture zones have already been identified in Victoria by the ECC report (ECC, 2000).
According to the Fisheries Act 1995, the management plan must include:
- The management objectives of the plan;
- Specify the management tools and other measures to be used to achieve the management objectives;
- Include guidelines for the criteria to be used in respect of the issue of licences and permits and in respect of the renewal, variation or transfer of licences;
- Identify critical components of the ecosystem relevant to the plan and current or potential threats to those components and existing or proposed preventative measures;
- Specify performance indicators, targets and monitoring methods including sample collection, analysis and reporting of data.
- Identify the biological, ecological, social and economic factors relevant to its management.
Given the above, the management plans for each designated aquaculture area should take the form of an impact assessment and include the following components:
- Identification of the aquaculture zone area, existing status, human uses and economic value;
- Baseline study to characterise the bathymetric, hydrographic, biological, water quality and ecological characteristics of the area;
- Summary of aquaculture species and production methods which are suitable for the zone (e.g. bivalves, gastropods, finfish), environmental impacts and estimated carrying capacity;
- Best practice management guidelines to address environmental issues;
- Licence conditions which should be imposed on prospective and existing aquaculturists, including baseline and routine environmental monitoring protocols;
- Research needs and priorities.
6.4 Characterisation Studies for Marine Aquaculture Areas
The size of the designated aquaculture zones ranges from 17 ha to 40 ha (landbased) and 20 ha to 1,000 ha (offshore). It is expected that the offshore aquaculture areas may be sub-divided into a number of leases. Due to the large area to be covered within each aquaculture zone, it would be impractical to conduct a baseline study of the zone that is comprehensive enough to be used to enforce compliance standards against individual operators in leased areas. The primary purpose of the aquaculture zone characterisation study should therefore be to provide a broad-brush summary of the various physical and ecological attributes within the aquaculture zone. More specific baseline data should be provided by the lease-holders as a condition of their aquaculture licence. As described earlier, specific sampling methods and regimes for baseline and monitoring studies will be detailed in subsequent documents developed specifically for this purpose. The objectives of the aquaculture zone characterisation study should therefore include:
- Characterisation of the physical, hydrographic, biological and ecological components of the aquaculture area and localised variations within the management zone;
- Identification of any areas within the zone which are sensitive to aquaculture development (e.g. seagrass beds; localised sediment accumulation areas);
- Identification of any potential risks to aquaculture operations (e.g. pollution sources, algal blooms, other users etc)
- Recommend appropriate species, production methods, intensity of production and management controls at the zone;
Much of the information required for the characterisation studies, particularly in Port Phillip Bay, has already been collected as part of the Port Phillip Bay Environmental Study, and in particular, the Pinnace Channel Aquaculture Zone baseline study (Hickman and Coleman, 1998, Coleman et al. 2000) and bathymetric study (Barry and Bailey, 2000). In aquaculture areas outside Port Phillip Bay, however, more intensive data collection may be required. The scope of the survey should take into account the expected seasonal variation as well as the size and potential impact of the proposed culture area.
The collation of long-term data on water quality variables which adversely affects aquaculture operations should be a primary goal of NRE Fisheries / MAFRI baseline monitoring operations for the marine aquaculture (particularly shellfish) areas. NRE Fisheries currently operates the Victorian Shellfish Quality Assurance Program (VSQAP) in conjunction with industry, the characterisation study should review current monitoring procedures and recommend further monitoring, if required.
Ideally, the size and intensity of shellfish culture area should be related to the physical and ecological condition of the proposed culture sites. Models have been developed to predict the carrying capacity of coastal systems for bivalve production (Ross and James, 2000; Proctor and Hadfield, 1996). These models essentially link hydrodynamic characteristics with primary production to estimate sustainable levels of mussel production. In practice, however, if shellfish are too intensively stocked for the area in which they are farmed, growth rates will be poor due to food scarcity. The feasibility of utilising the CSIRO model to estimate sustainable levels of mussel production in Port Phillip Bay and Western Port should be evaluated.
Similarly the carrying capacity of coastal environments for finfish culture may be estimated using mathematical models which relate the waste type and loadings from the site to the prevailing hydrographic conditions (see Henderson et al 2001). This approach allows the impacts of finfish cage culture to be evaluated prior to development
After the characterisation study for the aquaculture zone has been completed and the appropriate aquaculture species and carrying capacity determined, the individual leases within the aquaculture area may be allocated by a process which is yet to be determined. On the basis of this review of marine aquaculture management and the associated risk assessment, licence conditions for marine aquaculture in Victoria can be recommended as part of the management plan for the aquaculture area. This approach should allow a combination of self-monitoring and reporting by the industry which is underpinned by a Government monitoring compliance strategy.
6.5 Licence Conditions for Marine Aquaculture
After the baseline study for the aquaculture zone has been completed and the appropriate aquaculture species and carrying capacity determined, the individual licences/leases within the aquaculture area may be allocated by a process which is yet to be determined. On the basis of this current review of marine aquaculture management, and the associated hazard assessment, the recommended licence conditions for marine aquaculture in Victoria are outlined below. This approach allows a combination of self-monitoring and reporting by the industry which is underpinned by a Government monitoring compliance strategy. Full details of licence conditions will be determined in the management planning process.
6.5.1 Shellfish culture
It is recommended that shellfish culture licences for both existing and new licence/lease areas should incorporate the following conditions:
- General – the limits of the licence/lease area must be within a designated aquaculture area, clearly defined and the operator should contain his operation within that area. The species to be cultured should be stipulated and have approval under Translocation Policies.
- Navigation – the licence/lease area and culture infrastructure should be clearly marked and lit at night to avoid conflict with other users.
- Environmental carrying capacity – limits should placed on length of longline per ha of licence/lease area and all longlines are to be kept clear of the seabed.
- Environmental monitoring – baseline studies should be carried out by the licence/lease holder and monitoring should be undertaken as required.
- Operators should be members of the Victorian Shellfish Quality Assurance Program.
- Chemical use. Chemical types and quantities should be reported on an annual basis to NRE Fisheries. Marine and Freshwater Resources Institute 38
- Waste – wastes from harvesting and processing must be disposed of in a manner which does not affect the ecology of the marine environment. Waste management protocols must be developed by industry and approved by NRE Fisheries.
- Disease – protocols for disease notification and surveillance.
- Visual impacts – conditions should be imposed on colour, size and shape of buoys and netting and other floating equipment and other factors influencing visual amenity.
- Access.
- Controls relating to noise, debris, predator control etc.
- Reporting on an annual basis, the operator must report to Executive Director, NRE Fisheries details of production, stocking density, chemical use and environmental monitoring results.
6.5.2 Finfish culture
It is recommended that licences/leases for intensive finfish culture operations should incorporate the following conditions:
- General -the limits of the lease area must be within a designated aquaculture zone, clearly defined and the operator should contain his operation within that area. The species to be cultured should be stipulated and any other species will require a licence variation.
- Environmental carrying capacity – shall be determined prior to development using approved mathematical models and annual production limited accordingly. Stocking densities will be a maximum of 25 kg/m3 (salmonids and other species fed on commercial diets) and 4 kg/m3 for ranched species fed on trash fish.
- FCRs should be stipulated according to species and production system.
- Fallowing of cage sites if bubbles of hydrogen sulphide and methane are observed.
- Nets must be at least 1m clear of the seabed at low tide.
- Environmental monitoring - baseline studies should be carried out as specified by Executive Director, NRE Fisheries and regular monitoring as required.
- Waste. Wastes from harvesting, processing and fouling organisms must be disposed of in an acceptable manner.
- Disease. Diseases outbreaks must be notified to Executive Director, NRE Fisheries.
- Visual controls - standards will be set relating to farming structures and equipment.
- Conditions are stipulated with respect to excess noise, maintenance of equipment and predators.
- On an annual basis a report must be submitted to NRE Fisheries containing:
- records on total fish production, maximum biomass, fish feed usage and mortalities;
- List of names and quantities of chemicals used on site;
- Location, size and stocking rates of cages and fallow areas;
6.6 Recommendations For Monitoring Of Marine Aquaculture In Victoria
As described earlier, specific sampling methods and regimes for site characterisation, baseline and monitoring studies will be detailed in subsequent documents developed specifically for this purpose.
Unacceptable changes to the marine environment as a result of aquaculture can be avoided or ameliorated through an effective environmental management framework which regulates development and evaluates environmental impacts prior to granting permission to develop (GESAMP, 1996). Environmental assessment and monitoring effort should then be related to the scale of impact of a given aquaculture operation. The hazard assessment conducted during this review showed that compared with intensive finfish aquaculture, the potential for ecological change as a result of bivalve aquaculture is low and the proposed monitoring regime should reflect this. The key to minimising the environmental effects of aquaculture operations is by controlling the location, size and intensity of the farms and ensuring that the licensee adopts best practice management methods.
6.6.1 Initial monitoring requirements for aquaculture licence areas
The overall sensitivity of the site in relation to the proposed development should be apparent from the management plan characterisation survey. However, there may be local variations within individual lease areas which can affect the impact of an aquaculture operation. The aim of the licence area baseline study/initial monitoring survey should therefore be to provide a reference data set which can be used to (GESAMP, 1996):
- Design an appropriate monitoring program including the location of sampling and reference stations;
- Establish the conditions of the local ecosystem (which may include assessment of temporal and spatial variability) against which future change can be compared.
The scope of the survey should take into account the expected seasonal variation as well as the size and potential impact of the proposed facility. The recommended components of baseline studies for marine shellfish and finfish aquaculture in Victoria are shown in Table 6.2.
Table 6.2: Recommended parameters which should be included in Victorian marine aquaculture baseline studies (✔ = required; X = required if triggers exceeded).
| Shellfish | Finfish | |||
|---|---|---|---|---|
| Existing | New | New | ||
| Bathymetric survey | ✔ | ✔ | ✔ | |
| Hydrographic survey | ✔ | |||
| Video | ✔ | ✔ | ✔ | |
| Seabed characteristics/ Important habitat profile | ✔ | ✔ | ✔ | |
| Sediment Chemistry | ||||
| Visual | X | ✔ | ✔ | |
| Redox | X | ✔ | ✔ | |
| Organic content (C/N) | X | ✔ | ✔ | |
| Stable isotope analysis | X | ✔ | ✔ | |
| Particle size analysis | X | ✔ | ✔ | |
| Sediment Biology | ||||
| Biological/ benthos | X | ✔ | ✔ | |
Shellfish aquaculture (existing sites)
It is recommended that all existing shellfish aquaculture lease areas should be required to conduct a video survey on a transect through the lease area as part of a baseline survey. Licence/leaseholders must also submit a bathymetric map of their lease and a sketch showing seabed characteristics and important habitats. The video survey will be used to assess the condition of seabed beneath the culture lines and should be conducted beneath the longline with the heaviest stocking density. The exact location of the transect line will be lease-area specific and determined by NRE Fisheries in consultation with the lease-holder. If the video survey (and associated diver's log) shows signs of organic enrichment or debris beneath the culture lines, then further survey work should be undertaken. Protocols for sample collection, analysis and reporting of these parameters will be developed as part of the Management Plan process. Suitable control sites will be identified during the initial monitoring surveys. Specific triggers for further work include:
| Trigger | Action | |
|---|---|---|
| Organic Deposition |
|
|
| Debris |
|
|
Shellfish aquaculture (new sites)
For new shellfish aquaculture lease areas, baseline studies should collect physical, chemical and biological data on sediment quality so that there is data to assess the degree of disturbance in subsequent years. It is recommended that a video transect be marked out for 100 m through the centre of the lease area, or in a location determined by NRE Fisheries. Sediment samples should be collected at either end of the transect, as well as in the middle. Samples should be analysed for visual quality, redox, organic content, particle size and benthic invertebrates. Protocols for sample collection, analysis and reporting for these parameters will be developed as part of the Management Plan. A bathymetric map should be submitted along with a sketch of the important habitats in the lease area.
Finfish aquaculture
There is no existing offshore finfish culture in Victoria and any prospective culturists would be expected to undertake a comprehensive baseline study as part of an environmental impact assessment. Applicants would be required to submit a Consultants' report giving details of hydrographic monitoring, sediment and water quality at the proposed site together with technical details of the proposed development and the management practices to be employed on-site (Table 8.2). Protocols for sample collection, analysis and reporting of data will be developed as part of the Management Plan for zones where finfish aquaculture is permitted. The impact of the development on sediment quality should be assessed in the report using appropriate methods. The environmental impact assessment should consider the socio-economic impact of the development and methods to mitigate impacts which are identified.
6.6.2 Regular monitoring
There are various monitoring strategies that could be adopted to assess the impact of an aquaculture operation on the marine environment. The most appropriate strategy depends on the objective of the monitoring, viz:
- To ensure that impacts from the aquaculture site don't adversely affect the environment outside of the leased area (as required in Tasmania).
- To monitor the actual impact of production or fallowed aquaculture sites. This monitoring can validate the results of modelling and also provide feedback to farmers on the impact of their operation and farm management.
Since sediment impacts were highlighted as the most serious consequences of marine aquaculture in the hazard assessment, it is recommended that regular monitoring should focus on impacts on sediment quality (Table 6.3).
Analysis of water quality (eg. dissolved oxygen, ammonia, nutrients etc) should also comprise part of regular monitoring to be undertaken by the leaseholder.
Table 6.3: Recommended parameters which should be included in routine marine aquaculture monitoring ( ✔ = required; X = required if triggers exceeded).
| Shellfish | Finfish | ||||
|---|---|---|---|---|---|
| Frequency | Frequency | ||||
| Video | ✔ | Annual | ✔ | 6 month | |
| Sediment Chemistry | |||||
| Visual | X | If required | ✔ | Annual | |
| Redox | X | If required | ✔ | Annual | |
| Organic content (C/N) | X | If required | ✔ | Annual | |
| Stable isotope analysis Particle size analysis | X | If required | ✔ | Annual | |
| Biological | |||||
| Biological/ benthos | X | If required | ✔ | Annual | |
Shellfish culture
It is recommended that shellfish aquaculture lease areas should be required to conduct a video survey on an annual basis. The video survey will be used to make a preliminary assessment of the condition of seabed beneath the culture lines. The transect should either follow that originally marked out during the baseline study or it should be conducted beneath the longline with the heaviest stocking density. The appropriate location of the transect will be determined on a lease area basis by NRE Fisheries. If the video survey (and associated divers' log) shows signs of organic enrichment or debris beneath the culture lines, then further survey work should be undertaken. Specific triggers for further work include:
| Trigger | Action | |
|---|---|---|
| Organic Deposition |
|
Longlines should be moved and/ or stocking densities should be lowered at the site. |
| Debris |
|
Operator must remove |
Finfish culture
More stringent monitoring is required for finfish aquaculture (see Table 6.3). It is recommended that video surveys are conducted beneath production cage groups every six months. These surveys will be looking for evidence of serious organic enrichment as indicated by extensive mats of Beggiatoa sp. or outgassing from the sediments. The results of the 6 monthly survey will be used by the farm to modify management practices and reduce organic loadings in areas which show signs of waste accumulation. The results of the 6 monthly survey and the management measures adopted to address them should be recorded and submitted to NRE Fisheries with the annual survey. Water quality samples should be collected at least once per month and the results reported annually to NRE Fisheries.
The annual survey should comprise video surveys, sediment sampling and biological analysis under each productive cage group and control site(s). If unacceptable impacts are found then the lease holder and NRE Fisheries must work together to alter management practices at the site so that the impacts are ameliorated. Unacceptable impacts are defined as follows:
| Trigger | Actions | |
|---|---|---|
| Organic Deposition |
|
|
| Debris |
|
|
| Water Quality | Water quality to comply with objectives for environmental quality indicators as defined in the SEPP. |
|
Criteria for environmental quality standards to be met by marine aquaculture in Victoria will be detailed in subsequent documents which will outline site characterisation, baseline and monitoring requirements. All monitoring will include independent monitoring of control/reference sites to be undertaken by government, as well as independent review of procedures.
7 Appendix I: Hazard Assessment
This hazard assessment has been carried out according to the DNRE Risk Management Strategic Framework and Process which is based on the Australian/New Zealand Standard for Risk Management (AS/NZS 4360:1995).
7.1 Risk Identification
Environmental risks associated with bivalve shellfish, abalone and finfish culture were identified in Tables 5.1, 5.2 and 5.3, respectively. These tables are reproduced beneath in a Risk Management Framework
7.1.1 Likelihood
Likelihood ratings adopted for this risk assessment are as follows:
| Likelihood Rating | Description | Likelihood of Occurrence |
|---|---|---|
| 1 | Rare | Event may occur only in exceptional circumstances |
| 2 | Unlikely | The event may occur at some time, say once in 10 years |
| 3 | Moderate | The event should occur at some time, say once in 3 years |
| 4 | Likely | The event will probably occur in most circumstances, say once a year |
| 5 | Almost Certain | The event is expected to occur in most circumstances, say many times a month |
7.1.2 Consequence rating
The consequence of these risks with specific reference to environmental impacts are as follows:
| Consequence Rating | Description | Environmental |
|---|---|---|
| 5 | Catastrophic | Serious long-term or widespread environmental harm. |
| 4 | Major | Significant environmental harm with long-term recovery |
| 3 | Moderate | Moderate harm with mid-term recovery |
| 2 | Minor | Transient environmental harm |
| 1 | Insignificant | Brief pollution with effective remediation |
7.1.3 Risk ratings
| Risk Ranking | Score | Assessment |
|---|---|---|
| High | >8 | Requires detailed research, planning and decision making at senior levels of management |
| Significant | 7 | Senior management attention and action needed |
| Moderate | 6 | Management responsibility must be specified |
| Low | <5 | No major concern |
7.2 Bivalve Shellfish Culture
| Risk Category | Specific Risk | Description | Likelihood | Consequence | Risk Rating |
|---|---|---|---|---|---|
| Sediments | Deterioration in sediment quality | Can occur if organic wastes accumulate beneath culture lines (metabolic wastes) | 3 | 3 | 6 |
| Alteration of physical structure of sediments | Can occur if dead shells and other detritus are allowed to accumulate | 3 | 3 | 6 | |
| Water column | Depletion of essential nutrients | Can occur if culture area is not productive or if culture site overstocked | 2 | 2 | 4 |
| Modification of nutrient cycle | Can occur through transformation of plankton to excreta | 2 | 1 | 3 | |
| Reduction in dissolved oxygen levels | Can occur through transformation of plankton to excreta or if site over stocked | 2 | 1 | 3 | |
| Biological | Impacts on native bivalve populations through collection of seedstock | Can occur through over collection of wild spat | 1 | 2 | 3 |
| Impacts on seagrass beds | Competition for food | 3 | 3 | 6 | |
| Impacts on native fauna | Can be caused by cage infrastructure causing physical obstruction | 2 | 2 | 4 | |
| Coastal Resources | Sedimentation patterns | Large areas of culture infrastructure may interfere with direction and velocity of currents | 1 | 2 | 3 |
| Social conflict | May occur as a result of impeding navigation | 2 | 2 | 4 | |
| Amenity value | Loss of visual amenity | Untidy or poorly marked infrastructure | 3 | 2 | 5 |
| Loss of amenity | Noise or other impacts caused by servicing of sites | 3 | 2 | 5 |
7.3 Abalone
| Risk Category | Specific Risk | Description | Likelihood | Consequence | Risk Rating |
|---|---|---|---|---|---|
| Sediments | Deterioration in sediment quality | Can result from accumulated waste feed or faeces | 3 | 2 | 5 |
| Alteration ofsediment quality | Results from sediment scouring caused by cage infrastructure | 4 | 3 | 7 | |
| Water column | Eutrophication | Caused by nutrients released from wastes | 2 | 3 | 5 |
| Biological | Biodiversity impacts | Potential impacts of escaped hatchery-reared stock | 3 | 3 | 6 |
| Disease transfer | Potential for disease transfer to wild stock | 2 | 3 | 5 | |
| Coastal resources | Social conflict | Competition with abalone fishermen | 2 | 2 | 4 |
7.4 Finfish
| Risk Category | Specific Risk | Description | Likelihood | Consequence | Risk Rating |
|---|---|---|---|---|---|
| Sediments | Depletion of DO levels | Can result from accumulated wastes beneath cages | 4 | 3 | 7 |
| Alteration of sediment nutrient cycle | Can result from accumulated wastes beneath cages and reduced DO levels | 3 | 3 | 6 | |
| Alteration of benthic microbial and invertebrate community | Can result from accumulated wastes beneath cages | 5 | 3 | 8 | |
| Alteration of physical characteristics | Can result from accumulated wastes beneath cages | 3 | 3 | 6 | |
| Accumulation of therapeutic chemicals | Possible if chemicals are used on site | 3 | 4 | 7 | |
| Water column | Nutrient enrichment of coastal waters | Leaching of nutrients from wastes | 5 | 3 | 8 |
| Dissolved oxygen depletion | Can result from accumulated wastes beneath cages | 3 | 3 | 6 | |
| Increased ammonia levels | Can result from overstocked fish and/or accumulated wastes beneath cages | 3 | 3 | 6 | |
| Release of therapeutic chemicals | Consequences of some chemicals largely unknown. | 2 | 3 | 5 | |
| Biological | Biodiversity impacts | Could cause impacts if seedstock are wild-caught | 2 | 5 | 7 |
| Biodiversity impacts | Genetic pollution through interaction of escaped and wild fish | 3 | 2 | 5 | |
| Biodiversity impacts | Entanglement/ obstruction of native fauna | 4 | 2 | 6 | |
| Disease transfer | to wild populations | 2 | 4 | 6 | |
| Coastal zone | Social conflicts | Obstruction of navigation | 1 | 2 | 3 |
| Social conflicts | Access to fishing grounds | 1 | 2 | 3 | |
| Amenity value | Visual amenity | Untidy/ poorly planned sites | 3 | 2 | 5 |
| Amenity value | Noise from servicing sites | 4 | 2 | 6 |
8 References
APFA, 1998. Environmental Code of Practice for Australian Prawn Farmers. Australian Prawn Farmers Association. 36p.
ASIC, 1997. An Environmental Framework for Coastal Aquaculture in Australia. Part 2 – Case Studies. Australian Seafood Industry Council, Deakin, ACT.
Barg, U. C. 1992. Guidelines for the promotion of environmental management of coastal aquaculture. FAO Fisheries Technical Paper 328. Food and Agriculture Organization of the United Nations, Rome, 1992.
Barry, J. and Bailey, M. 1999. Bathymetric survey of the proposed aquaculture zone, Pinnace Channel, Port Phillip. Marine and Freshwater Resources Institute, Internal Report.
Brooks, K. M. 2001. An evaluation of the relationship between salmon farm biomass, organic inputs to sediments, physicochemical changes associated with those inputs and the infaunal response – with emphasis on total sediment sulfides, total volatile solids, and oxidation-reduction potential as surrogate endpoints for biological monitoring. 172 pp. Aquatic Environmental Sciences, 644 Old Eaglemount Road, Port Townsend, Washington, USA.
Brown, J. R., Gowen, R. J. and McLusky, D. S. 1987. The effect of salmon farming on the benthos of a Scottish sea loch. Journal of Experimental Marine Biology, 109:37-51.
Burke, C.M. (1995). Microprofiles of oxygen concentration in cores from Port Phillip Bay taken in January & February 1994. Dept. Aquaculture, University of Tasmania, Launceston. 45pp.
Chamberlain, J., Fernandes, T. F., Read, P., Nickell, T. D., and Davies, I. M. 2001. Impacts of bio-deposits from suspended mussel (Mytilus edulis L.) culture on the surrounding surficial sediments. ICES Journal of Marine Science, 58: pp In press.
Cohen, B.F., Currie, D.R. and McArthur, M.A. (1998). Epibenthic community structure in Port Phillip Bay, Victoria, Australia. Marine and Freshwater Resources Institute Internal Report No. 10. November 1998.
Coleman, N., Longmore, A. and Cohen, B. (2000). Baseline data for the Pinnace Channel Aquaculture Site. Marine and Freshwater Resources Institute Report No. 34. (Marine and Freshwater Resources Institute, Queenscliff).
Cromey, C. J., Nickell, T. D. and Black, K. D. 2001a. DEPOMOD modelling the deposition and biological effects of waste solids from marine cages.
Cromey, C. J., Nickell, T. D., Black, K. D., Provost, P. G. and Griffiths, C. R. 2001b. Validation of a fish farm waste resuspension model by use of a particulate tracer discharged from a point source in a coastal environment.
Dosdat, A. and de la Pomelie, C. 2000. Regulation and monitoring of marine aquaculture in France. J. Appl. Ichthyol., 16 (2000), 157-162.
DNRE, 1997. Victoria's Biodiversity: Directions in Management. Department of Natural Resources, Victoria.
DNRE, 2002. Port Phillip Bay Environmental Management Plan: Plan and critical programs to 2003. Department of Natural Resources and Environment, Victoria.
DPIWE, 1999. Marine Farming Development Plan. Furneaux Islands. Food, Agriculture and Fisheries Division, Department of Primary Industries, Water and Environment, Tasmania.
ECC, 2000. Marine, coastal and estuarine investigation: Final Report. Environment Conservation Council (Victoria), 250 Victoria Parade, East Melbourne VIC 3002.
EPA, 1999. Environmental guidelines for the freshwater salmonid farming industry. Final draft. Best practice environmental management series. Environmental Protection Authority, Victoria.
Evrick, A., Hansen, P. K., Anve, J., Stigebrandt, A., Johannssen, O. and Jahnsen, T. 1997. Regulating the local environmental impact of intensive marine fish farming I: The concept of the MOM system (Modelling – ongrowng fish farms – monitoring). Aquaculture, 158 (1-2), 85-94.
FAO, 2000. World review of fisheries and aquaculture. In: The State of World Fisheries and Aquaculture. www.fao.org.
FAO/NACA, 1995. Regional Study and Workshop on the Environmental Assessment and Management of Aquaculture Development (TCP/RAS/2253). NACA Envrionment and aquaculture development series No. 1. Network of Aquaculture Centres in Asia-Pacific, Bangkok, Thailand.
Fernandes, T. F., Miller, K. L. and Read, P. A. 2000. Monitoring and regulation of marine aquaculture in Europe. J. Appl. Ichthyol. 16 (2000), 138-143.
Fisheries Victoria, 1998 Victorian Aquaculture Strategy. Fisheries Victoria, Department of Natural Resources and Environment, Victoria.
Fleming, A. E. and Hone, P. W. (eds) 1996. Abalone Aquaculture: Proceedings of the 2nd International Symposium on Abalone Biology, Fisheries and Culture. Aquaculture (special issue) 195 pp.
Gavine, F. M., Rennis, D. S. and Windmill, D. 1996. Implementing environmental management systems in the finfish aquaculture industry. Journal of Chartered Institute of Water and Environmental Management. 10, p 341-347.
GESAMP, 1996. Monitoring the ecological effects of coastal aquaculture wastes. GESAMP (IMO/FAO/UNESCO-IOC/WMO/WHO/IAEA/UN/UNEP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection). Rep. Stud. GESAMP, (57): 38p.
Gill, C. 1997. World feed panorama. High cost of feedstuffs: Global impact, response. Feed International. January 1997. pp 6-16.
Gooley, G. J., De Silva, S. S., Hone, P. W., McKinnon, L. J. and Ingram, B. A. (2000a). Cage aquaculture in Australia: A developed country perspective with reference to integrated aquaculture development within inland waters. In: Liao, I. C. and Lin, C. K. (eds). Cage Aquaculture in Asia: Proceedings of the First International Symposium on Cage Aquaculture in Asia. Asian Fisheries Society, Manila and World Aquaculture Society – Southeast Asian Chapter, Bangkok, pp 21-37.
Gowen, R. J and Bradbury, N. B. 1987. The ecological impact of salmonid farming in coastal waters: a review. Oceanography and Marine Biology Annual Review, 25: 563-575.
Hansen, P. K., Evrick, A., Schaanning, M., Johannssen, P. Aure, J., Jahnsen, T. and Stigebrandt, A. 1997. Regulating the local environmental impact of intensive marine fish farming II: The monitoring programme of the MOM system (Modelling – Ongrowng fish farms – Monitoring). Aquaculture, 158 (1-2), 85-94.
Harris, G., Batley, G., Fox, D., Hall, D., Jernakoff, P., Molloy, R., Murray, A., Newell, B., Parslow, J., Skyring, G. and Walker, S. (1996). Port Phillip Bay Environmental Study Final Report. CSIRO, Canberra, Australia. 239pp.
Henderson, A. R. and Davies, I. M. 2000. Review of aquaculture, its regulation and monitoring in Scotland. J. Appl. Ichthyol. 16 (2000), 200-208.
Henderson, A. R., Gamito, S., Karakassis, I., Pederson, P. and Smaal, A. 2001. Use of hydrodynamic and benthic models for managing environmental impacts of marine aquaculture. J. Appl. Ichthyol. 17 (2001), 163-172.
Hickman, N. J. and Coleman, N. 1998. Pinnace Channel Aquaculture Area. Site characterisation and summary of background environmental information and monitoring capability. Fisheries Victoria, Department of Natural Resources and Envrionment.
Holmes, N. (1982). Scientific report of the epibiotic panel study, Supplementary Paper 3. In: R.N. Sandiford (ed.), The Heated Effluent Study for Victorian Coastal Waters, Final Report and Supplementary Papers. Marine Science Laboratories, Queenscliff, October 1982. pp 87-172.
Hone, P. and Fleming, A. 1997. Abalone. In: The New Rural Industries – a handbook for farmers and investors. Rural Industries Research and Development Corporation, Canberra.
Jorgensen, C. B. 1952. On the relationship between water transport and food requirements in some filter feeding invertebrates. Biological Bulletin, 103(3), 357-63.
Kaspar, H. F., Gillespie, P. A., Boyer, I. C. and MacKenzie, A. L. 1985. Effects of mussel aquaculture on the nitrogen cycle and benthic communities in Kenepuru Sound, Malborough Sound, New Zealand. Marine Biology. 85, 127-136pp.
Kautsky, N. and Evans, S. 1987. Role of biodeposition by Mytilus edulis in the circulation of matter and nutrients in Baltic coastal ecosystems. Marine Ecology Progress Series, 38: 201-212.
MacKenzie, L. 1998. Blowing the budget? Nutrient resources and the Malborough mussel crop. Seafood New Zealand, March 1998.
MacLeod, C. 1996. Environmental monitoring – Marine benthos. SALTAS R&D Report. 193-211, SALTAS, Hobart.
Maroni, K. 2000. Monitoring and regulation of marine aquaculture in Norway. J. Appl. Ichthyol. 16 (2000), 192-195.
McGhie, T. K., Crawford, C. M., Mitchell, I. M. and O'Brien, D. 2000. The degradation of fish-cage waste in sediments during fallowing. Aquaculture. 187, 351-366.
Napier, M. H. 1999. An investigation into the use of alternative aquafeeds in aquaculture – using the caged southern bluefin tuna industry as a case study. B. Sc. Project, School of Agriculture and Resource Economics, La Trobe University.
Naylor, R. L., Goldburg, R. J., Primavera, J. H., Kautsky, N., Beveridge, M. C. M., Clay, J., Folke, C., Lubchenco, J., Mooney, H. and Troell, M. 2000. Effect of aquaculture on world fish supplies. Nature. 405, pp 1017-1024.
NCC, 1989. Fishfarming and the safeguard of the natural marine environment of Scotland. Nature Conservancy Council: Scottish Headquarters, Edinburgh. 136p.
NCC, 1990. Fishfarming and the Scottish freshwater environment. Nature Conservancy Council: Scottish Headquarters, Edinburgh. 285p.
ORR, 1999. Review of regulatory arrangements in the Victorian aquaculture industry. Final Report. Office of Regulatory Review, Victoria.
O'Sullivan, D. and Dobson, J. 2000. Status of Australian Aquaculture in 1998/99. Austasia Aquaculture Trade Directory 2000, Turtle Press, Hobart, Tasmania, pages 3-16.
Pearson, T. H. and Rosenberg, R. 1978. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanography and Marine Biology Annual Review, 16: 229-311.
Pearson, T. H. and Stanley, S. O. 1979. Comparative measurement of the redox potential of marine sediments as a rapid means of assessing the effects of organic pollution. Marine Biology, 53: 371-379.
Pederson, P. B. 2000. Monitoring and regulation of marine aquaculture in Denmark. J. Appl. Ichthyol. 16 (2000), 144-147.
PIRSA, 1999. Tuna farming. www.pir.sa.gov.au.
Proctor, R. and Hadfield, M. 1996. Beatrix Bay: observations and models. Water and Atmosphere. 4 (4). 1996.
Read. P. A., Fernandes, T. F. and Miller, K. L. 2001. The derivation of scientific guidelines for best practice for the monitoring and regulation of marine aquaculture in Europe. J. Appl. Ichthyol. 17 (2001), 146-152.
Ritz, D. A., Lewis, M. E. and Ma Shen, 1989. Response to organic enrichment of infaunal macrobenthic communities under salmonid cages. Mar. Biol. 103, 211-214.
Rodhouse, P. G., Roden, C. M., Hensey, M. P. and Ryan, T. H. 1985. Production of mussels, Mytilus edulis in suspended culture and estimates of carbon and nitrogen flow: Killary Harbour, Ireland. The Journal of the Biological Association of the United Kingdom, 65: 55-68.
Ross, A. H. and James, M. R. 2000. A method for estimating potential bivalve culture production in sheltered coastal waters. Aquaculture Research.
Sanchez-Mata, A and Mora, J. 2000. A review of marine aquaculture in Spain: production, regulations and environmental monitoring. . J. Appl. Ichthyol., 16 (2000), 209-213.
SEPA, 1998. Regulation and monitoring of marine cage fish farming in Scotland – a manual of procedures. Scottish Environmental Protection Agency, Stirling, UK.
Souchu, P., Vaquer, A., Collos, Y., Landrein, S., Deslous-Paoli, J-M. and Bibent, B. 2001. Influence of shellfish farming activities on the biochemical composition of the water column in Thau lagoon. Mar. Ecol. Prog. Ser. 218: 141-152.
Tenore, K. R., Corral, J. amd Gonzalez, N. 1985. Effects of intense mussel culture on food chain patterns and production in coastal Galicia, N. W. Spain. ICES, C.M. 1985/F:62.
Victorian Coastal Council, 2001. Victorian Coastal Strategy. Draft Report, April 2001. Victorian Coastal Council.
Welcomme, R. 1996. Aquaculture and World Aquatic Resources. In: Baird, D. J., Beveridge, M. C. M., Kelly, L. A. and Muir, J. F. 1996 (eds). Aquaculture and Water Resource Management. Proceeding of a conference held at the University of Stirling 21-25 June 1994.
Weston, D. P. 1986. The environmental effects of floating mariculture in Puget Sound. College of Ocean and Fishery Science, University of Washington, Seattle, Washington.
Whitfield, M. 1969. Eh as an operational parameter in estuarine studies. Limnol. Oceanogr. 14, 547-558.
