Marine Biotoxin Management Plan
VICTORIAN MARINE BIOTOXIN MANAGEMENT PLAN
Fifth Edition
August 2015
Acknowledgements
This project was funded by Fisheries Victoria a Division of the Department of Economic Development, Jobs, Transport and Resources (Victoria).
The project team would like to thank the following people for their valuable input which facilitated the completion of this project:
Fisheries Victoria | Dr Peter Appleford (Executive Director Fisheries Victoria) John Mercer (Aquaculture Officer) Neil Hickman (Senior Scientist, Aquaculture Program, retired) Andrew Clarke (Manager Aquaculture) |
The University of Tasmania | Prof Gustaaf Hallegraeff (Assoc Professor in Aquatic Botany) |
Queensland Health Scientific Services | Dr Geoff Eaglesham (Senior Scientist) |
Cawthron Institute | Paul McNabb (Senior Scientist) |
State Chemistry Laboratories, Victoria | Dr Craige Trenerry |
Medvet Sciences, IMVS | Chris Murray (Laboratory Manager) |
Marlborough Shellfish Quality Programme | Helen Smale (Program Manager) |
Brenda Hay | AquaBio Consultants Ltd |
1 Amendments
1.1 Amendments
Amendments can be made to this plan by contacting the co-ordinator with the suggested changes and reasons for them.
Industry is responsible for the management of their seafood safety risks under the Seafood Safety Act 2003 and the Food Standards Code for seafood and is consequently responsible for any amendment to this plan. Industry should only amend the plan where the amendment to be made is consistent with an amendment to the ASQAP Manual and the relevant legislation, and is supported by relevant advice provided by a food safety expert and other relevant scientists.
To become part of this plan, amendments need to be issued with a covering letter. Amendments are identified by the issue number in the page header, by a vertical line in the left margin adjacent to the line(s) that has been changed and in the amendments record on page i. Amendments will be numbered in sequence.
1.2 Amendment Record
It is important this plan is kept up to date by the prompt incorporation of amendments and recording in the amendment table on page i.
To update the plan, remove the appropriate pages, destroy them and replace with the newly issued pages. Instructions will be included in the covering letter when amendments are issued and sent. File the covering letter at the back of the plan and sign off and date this page.
2 Acronyms and Glossary of Terms
2.1 Acronyms
ASP | Amnesic Shellfish Poisoning (Toxin: domoic acid) |
ASQAAC | Australian Shellfish Quality Assurance Advisory Committee |
ASQAP | Australian Shellfish Quality Assurance Program |
AZA | Azaspiracid |
AZP | Azaspiracid Shellfish Poisoning (Toxins: AZA-1. AZA-2, AZA-3) |
BTX | Brevetoxins |
C toxins | Di-sulphated saxitoxin analogues |
CEC | Commission of European Communities |
| DoA | Department of Agriculture |
DHHS | Department of Health and Human Services, Victoria |
DEDJTR | Department of Economic Development, Jobs, Transport and Resources, Victoria |
DSP | Diarrhetic Shellfish Poisoning (Poisons – OA, DTX 1-3, PTX ) |
DTX | Dinophysistoxin |
EE or Ecowise | Ecowise Environmental |
ELISA | Enzyme Linked Immuno-Sorbent Assay |
EPA | Environment Protection Agency, Victoria |
FSANZ | Food Standards Australia & New Zealand |
FSC | Food Standards Code |
GTX | Gonyautoxins |
HPLC | High Performance Liquid Chromatography |
LCMS/MS | Liquid Chromatography – Mass Spectrometry/Mass Spectrometry |
MAFRI | Marine & Freshwater Resources Institute |
VMBMP | Victorian Marine Biotoxin Management Plan |
MS | Mass spectrometry |
MU | Mouse Units |
NATA | National Association of Testing Authorities |
neoSTX | Neosaxitoxin |
NSP | Neurotoxic Shellfish Poisoning (Toxins: BTX) |
OA | Okadaic acid |
PPB | Port Phillip Bay |
PSP | Paralytic Shellfish Poisoning (Toxins: STX, GTX, neoSTX, C toxins etc) |
PTX | Pectenotoxins |
PTX-2-SA | Pectenotoxin-2-seco acids |
SSCA | State Shellfish Controlling Agency |
STX | Saxitoxins |
TSP | Toxic Shellfish Poisoning |
VMBMP | Victorian Marine Biotoxin Management Plan |
VSOM | Victorian Shellfish Operations Manual |
WES | WATER ECOscience |
WP | Western Port |
YTX | Yessotoxins |
mg/kg | Milligrams per kilogram |
ug/100g | Micro-grams per 100 grams |
2.2 Glossary of Terms
Authorised Officer | An officer authorised under the relevant legislation. |
Growing Area | A marine or enclosed body of water (for example: bay, harbour, gulf, cove, lagoon, inlet, estuary or river) in which commercial species of bivalve molluscs grow naturally or are grown by means of aquaculture. |
Harvesting Area | An area that has been designated by the Authority for the purpose of growing and harvesting commercial quantities of shellstock for human consumption and may include wildstock or aquacultured shellstock. |
Authority | The government entity having the legal authority to implement the Food Standards Code - Standard 4.2.1 Primary Production and Processing Standard for Seafood in Victoria. |
Fisheries Victoria | Means either Fisheries Victoria as an Authority of the State of Victoria or as a division of the Department of Economic Development, Jobs, Transport and Resources. It was, previously a division of the Department of Environment, and Primary Industries and before that the Department of Primary Industries. |
3 Introduction
3.1 Background
Some species of marine microalgae (phytoplankton) produce natural toxins which may accumulate in the tissues of filter feeding shellfish. Toxic shellfish poisoning (TSP) may result in humans that have consumed contaminated shellfish.
Within Victoria, four shellfish poisoning syndromes are potentially of concern:
- Paralytic Shellfish Poisoning (PSP)
- Amnesic Shellfish Poisoning (ASP)
- Diarrhetic Shellfish Poisoning (DSP)
Other possible TSPs that have not been detected in Victorian shellfish to date include
- Neurotoxic Shellfish Poisoning (NSP)
- Azaspiracid Poisoning (AZP)
- Yessotoxins
The potentially causative organisms of these poisoning syndromes are provided in Sections 8.6 and 8.7.
The presence of biotoxins in shellfish not only poses a health risk to consumers but may also adversely impact on the aquaculture industry by lowering consumer confidence in the harvested shellfish product. These risks can be managed by the Victorian Marine Biotoxin Management Plan (VMBMP).
The second edition of the Victorian Marine Biotoxin Management Plan was developed for the Victorian Shellfish Quality Assurance Program (VSQAP) by Ecowise Environmental (EE) in conjunction with the Department of Primary Industries (DPI), Fisheries Victoria.
A new shellfish quality assurance program, the Victorian Shellfish Operations Manual (VSOM) was developed in 2009. This third edition of the Victorian Marine Biotoxin Management Plan reflects the second edition and the changes resulting from the implementation of the VSOM. Ecowise Environmental was engaged by Fisheries Victoria to review the technical aspects of the VMBMP and to incorporate material provide by Fisheries Victoria relating to administration, Harvest Area closure and reopening, agency responsibilities and contacts relating to the inception of the VSOM.
The fourth edition has been updated by the Department of Economic Development, Jobs, Transport and Resources with assistance from Dr Steven Brett of Microalgal Services Pty Ltd.
3.1.1 History of Biotoxin Surveillance
A history of Victorian shellfish quality assurance phytoplankton and biotoxin surveillance is presented in Section 5.7 of the Australian Victorian Marine Biotoxin Management Plan for Shellfish Farming (Todd, 2001).
In summary, the Victorian Shellfish Quality Assurance Program was established in 1987 to provide for the safe harvest of blue mussels commercially harvested for the purpose of human consumption. At that time, it serviced four aquaculture zones in Port Phillip Bay (PPB) (Clifton Springs, Grassy Point, Dromana and Beaumaris) and another in Western Port (WP) (Flinders Bight).
Wildstock mussels from the Gippsland Lakes and scallops from PPB and Bass Strait have also been included in the VSQAP program in the past. The program was operated by the Marine and Freshwater Resources Institute (MAFRI) for Fisheries Victoria, and was funded entirely by the latter until it was discontinued at the end of 1996. The program collected surface water samples and tissue samples on a regular basis, analysing the samples for phytoplankton and biotoxin respectively. This sampling regime provided for the monitoring of toxic phytoplankton species and biotoxins in commercial shellfish harvest areas.
During the absence of a formal Government run shellfish quality assurance monitoring program in Victoria from July 1997 until August 1999, WES (now Ecowise) performed phytoplankton monitoring for the mussel industry, either as the Victorian Mussel Growers Association or as individual growers, contracted WES to conduct phytoplankton and biotoxin monitoring.
Ecowise Environmental performed all of the subsequent monitoring and reporting components of the Victorian Shellfish Quality Assurance Program (VSQAP), and prepared and reviewed the Victorian Marine Biotoxin Management Plan, under contract to Fisheries Victoria.
From 1990 till 2003 under the VSQAP, phytoplankton and tissue PSP testing has been performed fortnightly at each of the five harvesting areas in PPB and WP, except Beaumaris. Shellfish harvesting at Beaumaris, and consequently monitoring, ceased in March 2001 and this area is no longer authorised for the harvest of bivalve shellfish for human consumption. ASP testing has also been performed each fortnight at the Clifton Springs and Flinders harvesting areas. From 2004 until 2009, the frequency of routine biotoxin testing was reduced to monthly. As a result, the VSQAP has provided a significant database to support decisions in regard to biotoxin management and risk.
In order to classify two additional areas of water within PPB, monitoring has been performed at the Pinnace Channel harvesting area, located in central PPB, since December 2003 and the Mount Martha harvesting area, located in eastern PPB, since August 2006.
The Pinnace Channel harvesting area was formally incorporated into the VSQAP in July 2007 and its initial comprehensive sanitary survey was completed in 2009. The Mount Martha harvesting area was formally incorporated into the VSQAP in January 2008 and its initial comprehensive sanitary survey was completed in 2009.
In 2009 shellfish quality assurance in Victoria was transitioned to an industry managed program described in the Victorian Shellfish Operations Manual (VSOM) oversighted and regulated by Government. At that time the VSQAP monitoring program was reviewed and risk assessed resulting in a number of improvements in both safety and cost. Amongst a number of changes, fortnightly phytoplankton sampling was introduced to act as an early warning trigger for shellfish tissue biotoxin sampling.
Since 2014, two shellfish wild fisheries have been developed in Victoria: the pipi fishery at Discovery Bay, and the scallop dive fishery in Port Phillip Bay. The scallop fishery includes two harvest areas: the Pinnace Channel Scallop Harvest Area and the North-west Port Phillip Scallop Harvest Area. Marine biotoxin monitoring plans for these areas have been developed taking into account the available historic biotoxin data for the area and shellfish species, the environmental conditions at the harvest areas, and the requirement for data to support planned modification of the sampling plans in the future. These plans are documented in the on-going management plans associated with each harvest area, and approved by PrimeSafe. They incorporate fortnightly shellfish sampling of pipis and scallops, combined in the case of scallops with phytoplankton sampling in alternate weeks.
3.2 Aims and Objectives
The principal aim of the Victorian Marine Biotoxin Management Plan is to provide for the protection of shellfish consumers from the hazards of marine toxic shellfish poisoning (TSP) from the commercial harvesting of bivalve shellfish for human consumption from shellfish harvesting areas within PPB and WP in Victoria.
The following objectives have been established to meet this aim:
- The maintenance of a monitoring program using phytoplankton monitoring in conjunction with biotoxin testing of bivalve shellfish tissue. Phytoplankton monitoring is used to provide early warning of the presence of phytoplankton with the potential to contaminate shellfish with marine biotoxins. The results of this monitoring may be used to initiate biotoxin testing, and in some cases harvesting closures. Shellfish tissue biotoxin levels are used to make harvesting reopening and regulatory decisions.
- To document all procedures and contacts required to effectively manage incidents of shellfish biotoxin contamination.
- To facilitate the harvesting of shellfish which are free from marine biotoxins.
- To provide an effective and co-ordinated response to marine biotoxin events, minimising the risk of human illness.
- Ensure public awareness of shellfish biotoxin events while minimising potential adverse publicity to the shellfish industry.
- Maintain updated management protocols (contingency plans) to allow rapid and effective responses to marine biotoxin events.
3.3 Scope
The Victorian Marine Biotoxin Management Plan is designed primarily for the commercial aquaculture harvesting of bivalve shellfish from PPB and WP, areas for which extensive phytoplankton records exist. With some modifications, the VMBMP may be adopted for commercial wild shellfish harvesting if appropriate. There is evidence that various shellfish species may not bioconcentrate and metabolise particular biotoxins in the same manner. Hence, some review of biotoxin monitoring protocols may be required should additional shellfish species be grown and commercially harvested within PPB and WP.
3.4 Review
This Victorian Marine Biotoxin Management Plan will be reviewed as required to reflect changes to scientific and technical knowledge and the requirements of the Authority. In such cases an updated, numbered "version" will be issued, incorporating all amendments. Reviews shall only be undertaken by Fisheries Victoria with good knowledge of the Victorian Marine Biotoxin Management Plan, and the VSOM and its application in Victoria. This document is Edition 4 of the Victorian Marine Biotoxin Management Plan.
Upon issue of an updated version of the Victorian Marine Biotoxin Management Plan, all previous versions are to be destroyed or stored in such a manner that superseded documentation will not be available for use.
4 Requirements for a Victorian Marine Biotoxin Management Plan
Division 3 of standard 4.2.1 of the Primary production and processing standard for seafood requires Harvesting areas be subject to a Victorian Marine Biotoxin Management Plan prepared in accordance with the ASQAP Manual or other condition recognised by the Authority. The ASQAP Operations Manual (2006), specifications are that a biotoxin management plan must define:
- The responsibilities of all parties involved in the management plan
- Hydrographical details describing predominant currents and circulatory patterns
- Species of shellfish cultured/harvested
- Sample sites
- Sampling frequencies
- Sampling methods
- Methods of analysis for water and shellfish samples
- Laboratories used for sample analysis
- Alert level/s for toxic/potentially toxic algal species
- Potentially toxic algal species list
- Actions to be taken by the Authority when either alert levels are exceeded or toxins are found in shellfish below closure levels
- Closure procedures including closure criteria, notification of closures to marine farmers and relevant authorities, public announcements, management during closures, product recall
- Opening procedures including opening criteria, notification of opening to marine farmers and relevant authorities, public announcements, procedures for opening inactive or seasonal growing areas
- Case definitions of toxic syndromes
5 Administration
5.1 Legislation and Guidelines
A list of Federal and State legislation and guidelines that may be relevant to biotoxin management are provided below. For further detail, refer to the relevant document.
5.1.1 Federal
5.1.1.1 Legislation
- Food Standards Australia New Zealand Act 1991 and its subordinate Australian New Zealand Food Safety Code (the ANZFSC) and Standard 4.2.1 - Primary Production and Processing Standard (the PPPS)
- Export Control Act 1982 and its subordinate Export Control (Fish & Fish Products) Orders 2005 and the Export Control (Prescribed Goods General) Order 2005
- Australian Shellfish Quality Assurance Program (ASQAP) Operations Manual (2006)
- Australian and New Zealand Guidelines for Fresh and Marine Water Quality (2000)
- Health Act 1958.
- Fisheries Act 1995.
- Food Act 1984.
- Seafood Safety Act 2003.
- Environment Protection Act 1970.
5.1.1.2 Guidelines
5.1.2 State
5.2 Roles and Responsibilities
5.2.1 Fisheries Victoria
The following are the responsibilities of Fisheries Victoria.
- Issue licences authorising aquaculture activity and wild take under the Fisheries Act 1995.
- Maintain and revise this MBMP as required.
- Advise on the classification of harvesting areas and the revision of the MBMP and the VSOM.
- Oversight:
- industry sampling.
- opening and closures due to phytoplankton and or biotoxin.
- preparation of annual reports.
- Oversight comprehensive sanitary surveys and triennial reviews.
- Maintain databases for phytoplankton and biotoxin.
- Provide expert advice to industry.
- Provide representation at the national Australian Shellfish Quality Assurance Advisory Committee (ASQAAC).
- Provide in field observation and reporting of suspected compliance breaches
5.2.2 PrimeSafe
PrimeSafe is the authority responsible for administering the Seafood Safety Act (Victoria) 2003. The Seafood Safety Act 2003 requires seafood businesses (includes commercial bivalve shellfish harvesters for human consumption and bivalve shellfish processors) to be licensed and to have in place an approved seafood safety plan.
PrimeSafe's functions include:
- Control and review standards for construction and hygiene at seafood processing facilities.
- Licence seafood businesses including processing premises, harvesting vessels and vehicles handling seafood.
- Inspection of systems and audited quality assurance programs.
- Enforcement of necessary sanitary controls for processing plants and vehicles handling seafood.
- Detain and recall product considered unfit for human consumption.
- Regulate the processing of shellfish for human consumption by licensing, approval of food safety plans and auditing of compliance
- Implement the Food Standards Code for seafood in Victoria
5.2.3 Department of Health and Human Services (Victoria)
The following are the responsibilities of the Department of Health and Human Services (DHHS).
- Detain and recall product considered unfit for human consumption.
- Provide expert advice to the Authority.
- Licence food transport vehicles (subject to the Seafood Safety Act 2003).
- Maintain epidemiological data for notifiable diseases (including TSP cases).
5.2.4 Local Government
The following are responsibilities of Local government through the Food Act 1984.
- Licence relevant businesses to handle seafood (subject to the Seafood Safety Act 2003) (for example: supermarkets).
- Enforce necessary sanitary controls for processing plants and vehicles handling seafood.
- Provide advice concerning local sewage spills/events.
5.2.5 Department of Agriculture (DoA)
DoA is the Commonwealth government agency responsible for the administration of the export controls for seafood. The agency administers the export inspection system and provides certification for shellfish exports.
DoA administers the export inspection program, which includes provision for:
- The registration of premises, including vehicles, which prepare shellfish intended for export.
- The inspection of registered export establishments for implementation of good food processing practises.
- Conducting HACCP based food processing controls for exporters.
- Auditing state shellfish quality assurance programs for export accreditation and for compliance with the Export Control Act (Commonwealth) 1982 and its subordinate orders including the Export Control (Fish & Fish Products) Orders 2005.
DoA staff conduct compliance inspections and audits of land based shellfish processing establishment in accordance with the compliance history of the establishment and food safety risk associated with the food being prepared for export. The Export Control (Fish & Fish Products) Orders 2005 also regulate the controls for export of shellfish and shellfish handling, processing, purification, packing, storage, shipping, the labelling of shellstock to enable source identification and the recall, detention, seizure or destruction of shellfish unfit for human consumption for shellstock intended for export.
5.2.6 Shellfish Harvesting Industry
The following are the responsibilities of the industry who harvest bivalve shellfish for human consumption.
- Comply with the requirements of their PrimeSafe licence.
- Manage their seafood safety risks.
- Ensure no harvesting takes place when a closure is in place.
- Undertake a notification process, when required, for the recall of contaminated shellfish.
- Control the harvesting of shellfish based on sanitary conditions.
- Undertake the sampling program.
- Sub-contract components of the program to the private sector where required.
- Ensure no illegal harvesting takes place when a closure is in place.
- Retain records of closure and re-opening notices for harvesting areas.
- Retain records of monitoring, sampling and harvesting of harvesting areas.
- Provide representation at the national Australian Shellfish Quality Assurance Advisory Committee (ASQAAC).
- Provide expert advice to PrimeSafe, Fisheries Victoria, harvesters and the community concerning events adversely affecting water quality in PPB and WP.
- Provide guidance on shellfish safety and quality.
- Provide a set of guidelines for states and territories (the ASQAP Operations Manual).
- Be responsible for the formulation and regular updating of the ASQAP Operations Manual.
5.2.7 Environment Protection Authority (EPA Victoria)
5.2.8 Australian Shellfish Quality Assurance Advisory Committee
6 Hydrographical Details of Harvesting Areas
This Victorian Marine Biotoxin Management Plan has been prepared in respect of Harvesting Areas in the following geographical locations:
(i) Clifton Springs Harvesting Area
(ii) Dromana Harvesting Area
(iii) Flinders Harvesting Area
(iv) Grassy Point Harvesting Area
(v) Mount Martha Harvesting Area
(vi) Pinnace Channel Harvesting Area
(vii) Pinnace Channel Scallop Harvest Area
(viii) North-west Port Phillip Scallop Harvest Area
(ix) Discovery Bay Pipi Harvest Area.
The hydrographical details describing predominant currents and circulatory patterns are provided in the relevant sections of the management plans and referenced documents that cover each harvesting area:
Geelong Arm Aquaculture Fisheries Reserves Management Plan (2005):
- Clifton Springs Harvesting Area
- Grassy Point Harvesting Area
Eastern Port Phillip Bay Aquaculture Fisheries Reserves Management Plan (2005):
- Dromana Harvesting Area
- Mount Martha Harvesting Area
Flinders Aquaculture Fisheries Reserve Management Plan (2005):
Flinders Harvesting Area
Pinnace Channel Aquaculture Fisheries Reserve Management Plan (2005):
- Pinnace Channel Harvesting Area
Sanitary Survey Report for the Discovery Bay Growing Area
Proposal To Extend Classification Of Pinnace Channel Harvest Area To Harvest Of Scallops
Proposal For Classification Of Northwest Port Phillip Bay Scallop Harvest Areas
Detailed material may also be found in the following supporting documents all published as part of the Fisheries Victoria Report Series:
- Geelong Arm Aquaculture Fisheries Reserves – current, wind and wave data (2004)
- Environmental Characterisation of the Aquaculture Fisheries Reserves in the Geelong Arm, Port Phillip Bay, Victoria (2004)
- Eastern Port Phillip Bay Aquaculture Fisheries Reserves – current, wind and wave data (2004)
- Environmental Characterisation of the Aquaculture Fisheries Reserves in Eastern Port Phillip Bay, Victoria (2004)
- Environmental Characterisation of the Flinders Aquaculture Fisheries Reserve in Western Port, Victoria (2004)
- Baseline Data for the Pinnace Channel Aquaculture Site (2001)
- Pinnace Channel Fisheries Reserve - current, wind and wave data (2003)
- Bathymetric Survey of the Proposed Aquaculture Zone, Pinnace Channel Port Phillip (2001)
7 Species of Shellfish Cultured and Harvested
The species of shellfish covered by this Victorian Marine Biotoxin Management Plan are:
- Blue mussels, Mytilus galloprovincialis,
- Native oyster, Ostrea angasi
- Scallops, Pectin fumatus
Pipis, (Plebidonax deltoids) at Discovery Bay are covered by the Interim Biotoxin Management Plan for the Discovery Bay
8 Monitoring
8.1 Monitoring Program Goals
The Victorian Marine Biotoxin Management Plan provides a phytoplankton and biotoxin monitoring program that has been designed with the following goals in mind:
- Provide early warning of potential marine biotoxin contamination by detecting changes in the presence and abundance of potentially toxic phytoplankton species.
- Increase the knowledge and a wider understanding of the presence of those species that pose a potential marine biotoxin threat to commercial harvesters of shellfish for human consumption.
- Establish a long-term data set of phytoplankton abundance, marine biotoxin levels and events, and associated ecological factors. This dataset may be used to improve risk assessment, facilitate the analysis of trends in phytoplankton abundance and aid the prediction of marine biotoxin events.
- Provide toxic phytoplankton abundance trigger levels that permit harvesting closures in a timely manner before biotoxins reach levels that may threaten human health.
- Provide biotoxin levels to permit harvesting area closures and re-openings in a timely and safe manner.
- Validate that phytoplankton monitoring captures all toxic events where the risk assessment requires.
- Maintain an up to date list of local, national and international potentially toxic phytoplankton species.
8.2 Sampling Sites
When sampling sites for toxic phytoplankton and shellfish are established the following general factors were considered:
- The history of phytoplankton and marine biotoxin levels in PPB and WP.
- The need to monitor effectively the entirety of all aquaculture shellfish harvesting areas.
- Location of bivalve shellfish being harvested at various times.
- Accessibility of sample sites in various weather conditions.
- Environmental factors likely to influence sampling, such as:
- Major currents.
- Retention zones and circular patterns.
- Areas where algal blooms and fish kills were regularly observed, or had been regularly observed in the past.
- Impact of rivers.
- Impact of drains.
- Any other factors that may have influenced sampling.
- Sites have been chosen so that the water being sampled for phytoplankton is representative of the water being filtered by the shellfish within the Harvesting Area.
- For line culture, the water samples are collected so that the entire depth of the lines bearing shellfish is sampled, to account for the possibility of uneven vertical distribution of phytoplankton.
The aquaculture harvesting areas within PPB and WP are all in open, well circulated and vertically mixed waters. Shellfish and phytoplankton are sampled where suitable shellfish are available and where harvesting for human consumption is to occur. Consequently, sampling maybe carried out at different sites within each harvesting area over consecutive sampling events.
8.3 Sampling Officers and Sample Collection
It is a NATA requirement that sampling be undertaken by appropriately trained personnel. In addition, a suitably trained Fisheries Victoria staff member will be available to undertake training of samplers and the provision of advice when required.
All sampling must be performed in accordance with the sampling protocols provided in this Victorian Marine Biotoxin Management Plan.
Sample collection forms as provided in the VSOM must be completed with each sample event. These provide a chain of documentation of any observations made within the harvesting areas, such as weather conditions, or anything else that may be relevant to the sample collection process and sample integrity. Industry must retain a copy of the sample collection forms on file. All sample collection forms are made available to the PrimeSafe and its nominated auditor upon request.
Where scheduled samples cannot be collected during any sampling event, this is recorded in the auditable documentation and reported to the Authority (site and reason) as soon as possible.
8.4 Sampling Safety
It is the responsibility of the sampler and boat master to ensure that all sampling is undertaken in a safe manner that does not endanger human safety and is consistent with all legislative requirements.
8.5 Phytoplankton Monitoring
8.5.1 Sampling Frequency
Since 1987, phytoplankton sampling has been carried out at all harvesting areas within PPB and WP, usually on a fortnightly basis. Consequently, a considerable body of data exists concerning phytoplankton blooms in these waters.
Phytoplankton sampling is carried out in all harvesting areas on a fortnightly, routine basis during harvesting times, which is normally all year round. The frequency of sampling has been found to be adequate to allow phytoplankton monitoring to provide early warning of the potential for biotoxin contamination of bivalve shellfish tissue and as a trigger to initiate tissue sampling for biotoxin analysis. The determination of the presence or absence of potentially biotoxin-producing phytoplankton in water samples is undertaken consistent with the requirements of the Food Standards Code and the Export Orders.
Where phytoplankton monitoring reveals the presence of potentially toxic phytoplankton species in numbers equal to or above the trigger levels then biotoxin testing must be undertaken.
Where phytoplankton monitoring reveals the presence of potentially toxic phytoplankton species in numbers approaching the trigger for biotoxin testing, or in rising numbers, the sampling frequency should be increased to monitor the state of the bloom. Once the bloom has degenerated the sampling frequency may be reduced.
8.5.2 Sampling Methods
Detailed instructions for the use of appropriate phytoplankton sampling equipment is presented in Appendix 6.
Two samples, a concentrated plankton net haul and a depth-integrated hosepipe sample, are collected for phytoplankton analysis:
- A concentrated sample using a 6m vertical haul with a 20µm mesh plankton net; this sample is used to identify any potentially toxic or nuisance species present, particularly those with very low abundance trigger levels. Although there is the potential that fragile algae such as the non-armoured gymnodinioids may be damaged by the use of these nets, experience has shown that these cells are sampled intact when the nets are used appropriately.
- The concentrated net sample is collected in a 75mL polycarbonate vial attached to the net, and transferred to a separate, larger storage bottle leaving a 20 – 30 mm air space, and capped tightly. The sample is labelled appropriately with the date and time of sampling, sample type and the Harvesting Area.
- A depth-integrated sample is collected using a 6 m long, 25mm internal diameter hosepipe sampler and placed into a clean bucket on board the sampling vessel. This is mixed thoroughly taking care to avoid damage to any phytoplankton present and a 1 L sub-sample collected for enumeration. A 20 – 30 mm air space left prior to capping. The sample is labelled with the date and time of sampling, sample type and the Harvesting Area.
- All samples are collected so that foreign inclusions are avoided (e.g. outboard motor oil).
- All samples are stored in an upright position in an esky containing a small ice pack that does not contact the samples – the purpose of the ice-pack is solely to prevent the interior of the esky from heating up and not to cool the sample(s).
- Excess shaking of the samples during transport and sampling is to be avoided as this may damage some phytoplankton.
8.5.3 Laboratory used for phytoplankton analysis
It is a requirement of the VMBMP and the VSOM that the analysis of all phytoplankton samples be undertaken at NATA registered laboratories or international laboratories with quality assurance programs of equivalent standard. Appendix 2 lists the name and contact details of organisations that, at the time of writing, may be used to provide analytical services for the identification and enumeration of phytoplankton.
8.6 Phytoplankton Species Monitored
Appendix 7 contains lists of phytoplankton species present or likely to be present in Australian waters sorted into the following categories on the basis of their likelihood of occurrence and potential for toxicity:
- Category A1 - Species known to be present in southern Australian waters including PPB and WP, and proven or suspected toxin producers in Australia.
- Category A2 - Species known to be present in Australian waters and proven to produce toxins in Australia or overseas.
- Category B - Potential toxin producing species (i.e. toxicity untested/unclear) known to be present in Australian coastal waters.
- Category C - Other potential toxin producing species worldwide that may be present in Australian waters.
The phytoplankton monitoring program must at all times be able to identify potentially toxic species on these lists, particularly those in Categories A & B. In some cases, where species identification is difficult, or the taxonomy is unclear, similar species may be managed as a single group. For example, despite the fact that only two species encountered have a record of toxicity, all Pseudo-nitzschiaspp. are initially assumed to be as toxic as the most toxic member of the group. This allows for conservative management until definitive identification is made. This principle is also applied to any case where the identification of a potentially toxic species is uncertain.
Appendix 9 lists the trigger levels for phytoplankton species within PPB and WP. These relate to enumeration using an integrated phytoplankton sample collected with a hosepipe sampler. These triggers are used to initiate tissue biotoxin testing and precautionary harvesting closures pending biotoxin results.
8.7 Tissue biotoxin monitoring
8.7.1 Sampling Frequency
Aquaculture Harvest Areas
- PSP biotoxin tissue analysis is carried out when potentially toxic phytoplankton abundance levels indicate this is necessary.
- Until January 2006, ASP (domoic acid) biotoxin tissue analysis was carried out routinely every four weeks at the Clifton Springs and Flinders harvesting areas, to provide background data for the two major bays where aquaculture harvesting areas exist. From that date, analyses are performed when potentially toxic phytoplankton abundance levels indicate this is necessary. Domoic acid has not been recorded in mussels from any harvesting area.
- Other biotoxin analyses (DSP, NSP, AZP) are performed when potentially toxic phytoplankton abundance levels indicate this is necessary.
Scallop Harvest Areas:
As a minimum, sampling frequency meets the requirements specified for aquaculture areas. Currently additional scallop sampling is undertaken as per the management plans for each harvest area.
Pipi Harvest Areas:
As a minimum, sampling frequency meets the requirements specified for the Discovery Bay pipi area. Currently additional pipi sampling is being undertaken as per the management plan for that harvest area.
8.7.2 Shellfish Species Sampled
The shellfish species sampled for marine biotoxin analysis are those that are harvested for human consumption. Currently this includes: Blue mussels, Mytilus galloprovincialis ; Commercial scallop Pecten fumatus (Pinnace Channel and North-West Port Phillip Scallop Harvest Areas).
The tissue portions to be analysed must match the product that is to be marketed i.e. whole tissues for mussels, oysters, pipis and scallops, unless only the scallop muscle tissue and roe are supplied to the market, in which case muscle and roe only are tested.
8.7.3 Methods
8.7.3.3 Sampling
- For areas where the risk rating is unknown due to insufficient historical data shellfish are collected routinely for biotoxin analysis.
- For areas where the risk is assessed as low shellfish are collected for tissue biotoxin analysis when phytoplankton samples are approaching or exceeding early warning alert levels at a Harvesting area.
- For each mussel biotoxin analysis, 30 - 40 large mussels are required from each site sampled. For scallops, 25 market-sized scallops are required. (Mussels are used as indicator species for oysters.) Mussels are shucked in the laboratory and 150 – 180 g flesh prepared for each biotoxin analysis.
- Shellfish should be transported after collection in eskies containing ice packs to keep them cool. Shellfish are not to be frozen or cooled excessively.
8.7.3.4 Laboratory Testing
Analytical laboratories undertaking marine biotoxin analysis of shellfish samples must be NATA-accredited (or for overseas laboratories, an equivalent accreditation) for the tests undertaken. Advanced Analytical Australia, a NATA certified laboratory in Sydney can perform the full range of biotoxin analyses required by the Food Standards Code which includes PSP, ASP, DSP and NSP, plus analysis for additional toxins such as azaspiracids and yessotoxins if required. Appendix 3 lists the organisations that can provide analytical services for biotoxin analysis of shellfish tissue samples. Details of the methodologies used are provided in Appendix 5.
There are four main groups of toxins of concern within Australia that may accumulate in shellfish tissue and cause illness in humans. These are named after the poisoning syndrome they cause. The regulatory limits applied within the Victorian Marine Biotoxin Management Plan meet and in some cases are more conservative than those of the FSANZ Food Standards Code (FSC).
Paralytic Shellfish Poisons (PSPs)
A range of Paralytic Shellfish Toxins such as STX, C toxins and gonyautoxins are produced by several dinoflagellate species including Alexandrium catenella, A. minutum, A. tamarense and Gymnodinium catenatum. These toxins may be fatal to human consumers of contaminated shellfish through respiratory paralysis, although this is rare and there have been no fatal cases in Australia. PSP was detected in PPB mussels in 1993 and 1994 at the Clifton Springs and Grassy Point harvesting areas; the most likely source was A. tamarense (Arnott et al, 1999). The maximum PSP concentration detected was 276 mg/100g at Clifton Springs.
Current testing:
Testing Agency for PSP: Advanced Analytical Australia Pty Ltd , New South Wales.
Method: PST screening by LC-FLD (Lawrence Method) PST confirmation by LC-FLD AOAC 2005.06 (Lawrence Method)
Units: mg/kg (or mg/100g)
FSC Regulatory Limit: 0.8 mg/kg (STX Equivalent)
Amnesic Shellfish Poisons (ASPs)
Amnesic shellfish poisoning is caused by domoic acid produced by several species of diatoms belonging to the genus Pseudo-nitzschia, such as P. australis and P. multiseries. ASP may cause symptoms from nausea, vomiting and abdominal cramps to dizziness, hallucinations, short-term memory loss and seizures. Although most species of Pseudo-nitzschiaare non-toxic, they are very difficult to separate definitively using only light microscopy. Hence, all Pseudo-nitzschiaare initially assumed to be toxic until definitive identification is made. There are no documented cases of amnesic shellfish poisoning in Australia. Domoic acid has not been detected in Victorian mussels but has been detected in scallops from Bass Strait (Arnott et al, 1999).
Current testing:
Testing Agency for ASP: Advanced Analytical Australia Pty Ltd , New South Wales.
Method: LCMSMS (McNabb, P., Selwood, A.I.,Holland, P.T. (2005). J. AOAC Int. 88(3), 761-772.)
Units: mg/kg (ormg/g)
FSC Regulatory Limit: 20 mg/kg (Domoic Acid Equivalent)
Diarrhetic Shellfish Poisons (DSPs)
A range of DSP toxins such as OA, DTX 1 – 3 and PTX are produced by several species of dinoflagellate including Dinophysis acuminata, D. acuta, D. fortii and Prorocentrum lima. Diarrhetic shellfish poisons may cause gastrointestinal problems including diarrhoea, vomiting and abdominal pain; recovery occurs within 3 days irrespective of medical treatment (Hallegraeff, 1997). There have been no reported cases of diarrhetic shellfish poisoning within the areas covered by the Victorian Marine Biotoxin Management Plan.
In the past, PTX seco-acids have been included as a DSP toxin. However, subsequent work in New Zealand (MacKenzie 2002) for the Marlborough Sounds Shellfish Quality Program and within Australia (Burgess 2002) has shown that these compounds are not toxic to humans. Consequently, they are no longer regulated as a DSP toxin. Most of the "DSP" found in mussels tested from PPB during Dinophysis acuminata blooms, was PTX-2-SA.
DSP toxins are not defined within the ANZFSC, Standard 1.4.1 but, as noted in the ASQAP Operations Manual, are by both the European Union (Directive 2002/225/EC) and New Zealand Specifications for Bivalve Molluscan Shellfish. The following are included:
- Okadaic acid (OA)
- Dinophysis toxins (DTX)
- Pectenotoxins (PTX)
The FSANZ Food Standards Code Regulatory Limit for DSP is 0.2 mg/kg. However to meet internationally accepted levels the Victorian Marine Biotoxin Management Plan specifies a maximum limit of 0.16 mg/kg.
Current testing:
Testing Agency for DSP: Advanced Analytical Australia Pty Ltd , New South Wales.
Method: LC-MS/MS (McNabb, P., Selwood, A.I., Holland, P.T. (2005). J. AOAC Int. 88(3), 761-772.)
Units: mg/kg (ormg/100g)
FSC Regulatory Limit: 0.2 mg/kg (Okadaic Acid Equivalent) (Total of all DSP toxins), maximum limit applied 0.16 mg/kg (OA equivalents).
Neurotoxic Shellfish Poisons (NSPs)
Neurotoxic shellfish poisoning is caused by brevetoxins produced by some dinoflagellates, particularly Karenia brevis. NSP symptoms vary from headaches, diarrhoea, muscle and joint pain, and vomiting in mild cases, to paraesthesia, altered perception of hot and cold and breathing and swallowing difficulties in extreme cases. Which species produce BTX (brevetoxins) at levels sufficient to cause human intoxication is confounded somewhat by a lack of knowledge of the taxonomy of this group. The only suspected NSP incident in Australia was reported in 1994 and resulted from the consumption of wildstock mussels from the Tamboon Inlet in Gippsland, Victoria. K. cf brevis was identified as the organism responsible (Arnott, 1998).
Current testing:
Testing Agency for NSP: Advanced Analytical Australia Pty Ltd, New South Wales.
Method: LC-MS/MS
Units: mg/kg
FSC Regulatory Limit: 200 MU/kg or 0.8 mg/kg BTX-2 eq
Other toxins
Yessotoxins (YTXs)
YTXs and their derivatives have a structure similar to that of brevetoxins but do not have the same neurological effects. YTXs and their analogues appear to be produced by a number of dinoflagellates including Protoceratium reticulatum and Coolia monotis (Hallegraeff, 2002). YTX was detected in PPB in August 2011 associated with the algae Dinophysis acuminata with maximum levels of 0.027 -0.035 mg/kg. P. reticulatum has been found in most Harvesting Areas in Port Phillip Bay since 2011.
YTX is not regulated in Australia through the Food Standards Code and although it is toxic to mice when applied intraperitoneally, its oral toxicity is questionable (Cawthron Institute, 2001). The 32nd Session of the CODEX Committee on Fish and Fishery Products (1-5 October 2012) confirmed the exclusion of yessotoxins from the list of marine biotoxins that should be tested at international level. However, on 16 Aug 2013 the European Commission's European Food Safety Authority (EFSA) adopted an Opinion of the Scientific Panel to increase the limit to 3.75mg/kg. (Official Journal of the European Union, COMMISSION REGULATION (EU) No 786/2013).
Testing facilities for yessotoxin in shellfish are available.
Testing Agency for yessotoxins: Advanced Analytical Australia Pty Ltd , New South Wales.
Method: LC-MS/MS (McNabb, P., Selwood, A.I., Holland, P.T. (2005). J. AOAC Int. 88(3), 761-772.)
Units: mg/kg (ormg/100g)
Regulatory Limit: Not regulated in Australia, maximum limit applied in Victoria Marine Biotoxin Plan = 3.75 mg/kg.
Azapiracids (AZA)
Azaspiracid Shellfish Poisoning (AZP) is caused by a group of toxins with a novel chemical structure, called azaspiracids. AZP has occurred in Ireland and the symptoms include nausea, vomiting, diarrhoea and stomach cramps. The causative agent appears to be some strains of the dinoflagellate Protoperidinium crassipes(Hallegraeff, 2002). AZPs have not been detected in Australia or New Zealand.
AZA is not regulated in Australia through the Food Standards Code. The European Guidelines recommended a limit of 16 mg/100g for AZA equivalents.
Testing Agency for AZA: Advanced Analytical Australia Pty Ltd , New South Wales.
Method: LC-MS/MS (McNabb, P., Selwood, A.I., Holland, P.T. (2005). J. AOAC Int. 88(3), 761-772.)
Units: mg/kg (ormg/100g)
Regulatory Limit: Not regulated in Australia, maximum limit applied in Victoria Marine Biotoxin Plan = 0.16 mg/kg.
8.8 Environmental Information
At the same time as phytoplankton/biotoxin sampling is carried out, salinity, water temperature and the occurrence of rainfall local to the Harvesting Area are also recorded.
8.9 Reporting and Notification
- Results from the phytoplankton analyses are provided to the relevant Harvest Area Coordinator (HAC) and shellfish farmers and Fisheries Victoria within 24 hours of receipt. If analytical results reveal the presence of toxic phytoplankton species in significant numbers, the relevant HAC and shellfish farmers must be informed immediately via phone and e-mail.
- Biotoxin results are emailed to the relevant HAC and shellfish farmers when analysis is complete (2 - 4 days depending on the analysis required and day of sampling).
- If biotoxins are detected above the limit, the laboratory concerned notifies the HAC and Fisheries Victoria immediately. The HAC then immediately notifies the relevant shellfish farmers (or their delegate) by phone and e-mail to inform them of the result, allowing appropriate management action to be taken promptly.
- Should biotoxins be detected in shellfish tissue, it is the responsibility of the appropriate HAC to notify the relevant shellfish farmers (PrimeSafe licence holders), Fisheries Victoria, PrimeSafe and other relevant industry personnel and stakeholders (see Appendix 1). This should be done immediately by telephone and written confirmation provided by e-mail as soon as practicable.
- The approximate schedule for receiving laboratory results is displayed in Table 2.
Table 1: Approximate schedule for receiving routine sampling results
Results | Days | Methods |
Sampling | 0 | |
Phytoplankton Identification/enumeration | 1 | |
DSP, ASP, PSP, NSP, AZP Analyses | 2-4 |
- All reports issued contain comments explaining the significance of any "positive" results obtained and recommend management actions where appropriate.
The relevant authority contacts are presented in Appendix 1.
8.10 Data Storage
- Electronic and hardcopy reports of all analytical results must be maintained (stored) in a secure location, by the Harvest Area Cordinator , Fisheries Victoria and PrimeSafe licence holders on their data storage and filing systems, together with a copy of the field sampling sheet.
- Once all analytical results relating to a sampling event are received, the data are to be stored permanently on the Harvest Area Cordinators', Fisheries Victoria and PrimeSafe licence holders' databases.
- Fisheries Victoria Biotoxin database has been maintained by Fisheries Victoria since the inception of the VSQAP in 1987 and contains all monitoring data from that date until the end of March 2009. At this time industry was provided with a copy of that database and from that time the Harvest Area Cordinator and PrimeSafe licence holders storage systems are to retain all biotoxin data. Fisheries Victoria also continues to maintain the database for all Harvesting Areas.
8.11 Contingency Plans for Marine Biotoxin Events
Contingency plans (management protocols) for each of the known nuisance/toxic species encountered or likely to be encountered in PPB or WP have been formulated. These are attached in Appendix 11.
Each protocol contains the following:
- Title noting phytoplankton species to which it refers.
- Background information concerning the phytoplankton concerned, including toxicity.
- Rationale for the protocol.
- Step by step contingency plan.
- Details of the relevant abundance triggers for tissue testing and harvest suspension.
- Details of the regulatory limits for the relevant toxins.
Management protocols have been prepared for:
- Alexandrium spp.
- Pseudo-nitzschia spp.
- Dinophysis acuminata, Dinophysis spp., Prorocentrum lima
- Gymnodinium catenatum
- Karenia /Karlodinium group
- Azadinium spp.
- Rhizosolenia cf chunii
These contingency plans will be implemented in any of the following events:
- The abundance of any potentially toxic phytoplankton species exceeds the relevant trigger levels for biotoxin testing listed in Appendix 11.
- The detection of any phytoplankton species at levels known to be toxic overseas but of unknown toxicity in Australian waters.
- The presence of biotoxins in shellfish flesh.
- Any other reason as determined by the Harvest Area Cordinator or PrimeSafe.
The contingency plans will be reviewed and updated annually, or immediately if any relevant new information or regulation relating to marine biotoxins in shellfish becomes available. Advice to Harvest Area Cordinator and harvesters could be provided by PrimeSafe, contracted laboratories and consultants, the ASQAAC or other suitable qualified experts.
9 Area Closure and Reopening
9.1 Closure Criteria
The following criteria determine whether a closure needs to be implemented:
- The abundance of potentially toxic phytoplankton species exceeds the trigger for harvest suspension pending toxin analysis (as well as that for the initiation of biotoxin analysis) as noted in Appendix 9.
- The abundance of potentially toxic phytoplankton species has not yet exceeded the warning trigger level for biotoxin testing but is approaching that level, the precautionary principle must be applied and shellfish must be sampled for biotoxins.
- Biotoxins are present in shellfish at levels equal to or over the regulatory limits noted in Appendix 10.
- Confirmed or probable cases of human illness consistent with the case definitions for PSP, NSP, DSP and ASP (Appendix 8) have resulted from the consumption of shellfish from a particular harvesting area.
- PrimeSafe, as regulator of the Food Standards Code in Victoria in respect of seafood, or the PrimeSafe Licence holder determines a closure is necessary for any other reasons (e.g. potential toxin producing phytoplankton species which have not previously been recorded are present).
9.2 Mechanism for Closure
The following procedure is useful for the closure of a harvesting area
- The Harvest Area Cordinator will close a harvesting area and PrimeSafe licence holders must cease the movement of all shellfish immediately, if any of the closure criteria mentioned above are met.
- The closure area will extend to all of the harvesting area concerned.
- Closures should be made on a precautionary shellfish species-specific (to those grown in the harvest area) basis due to differences in the abilities of various shellfish to accumulate toxins. Where several species are involved, each should be tested to determine tissue toxin levels.
- Where harvesting is suspended in a harvesting area, a closure notice will be issued within 24 hours by the Harvest Area Cordinator and communicated (fax, post, e-mail or phone) to the following:
- All PrimeSafe licence holders that participate in the shellfish harvest monitoring program for the relevant harvesting area(s).
- PrimeSafe.
- Where the presence of biotoxins in shellfish tissue is confirmed, the public will need to be informed. Public warnings will be issued by the Public Health Division, Department of Health and Human Services based on advice provided by PrimeSafe.
- A recall of commercial product will be made where necessary by PrimeSafe (Refer to Section 11).
9.3 Industry Instigated Closure
PrimeSafe licence holders may choose to instigate a voluntary closure based on criteria such as pending biotoxin testing results, toxins present in neighbouring harvest areas, rising levels of toxic phytoplankton, the presence of Rhizosoleniacf chunii (bitter taste alga) or any other criterion they deem important enough to necessitate a closure.
9.4 Re-opening Criteria
- A shellfish harvesting area closed due to the presence of potentially toxic or unknown phytoplankton pending biotoxin analysis, may be reopened by the Harvest Area Cordinator immediately if the results of biotoxin testing prove negative.
- A shellfish harvesting area closed due to marine biotoxins shall not be reopened until the Harvest Area Cordinator has determined that each of the following requirements for reopening have been adequately addressed:
- The edible portion of each molluscan species harvested from the closed harvesting area meets the following criteria:
- PSP levels are less than the regulatory limit of 0.8 mg saxitoxin equivalent /kg edible shellfish flesh (80µg/100g) as determined Liquid Chromatography Fluorescence Detection (LC-FLD) in two successive samples from the same site taken at least 48 hours apart; phytoplankton abundance not rising.
- ASP levels are <10 mg domoic acid equivalent/kg edible shellfish flesh (20 µg/g or 20 ppm), by Liquid chromatography–mass spectrometry- mass spectrometry (LC-MS/MS) , in two successive samples from the same site taken at least 14 days (two weeks) apart; phytoplankton abundance not rising.
- DSP levels (not including pectenotoxin 2 seco-acids and their derivatives in mussels) are less than 0.2 mg okadaic acid equivalents/kg edible shellfish flesh (20 µg/100g) by LC-MS/MS, two successive samples from the same site taken at least 48 hours apart; phytoplankton abundance not rising.
- NSP levels are less than 200 mouse units/kg edible shellfish flesh (20MU/100g), by ether extraction and mouse bioassay with a maximum observation time of 6 hours, in two consecutive samples from the same site, taken not less than 48 hours (2 days) apart.
- The abundance of toxic phytoplankton relating to the toxin present has shown a clear downward trend and the cell counts are below the threshold level used to initiate closure (Appendix 9). The Harvest Area Cordinator and PrimeSafe licence holders should consider whether the level of other potentially toxic phytoplankton species are increasing, necessitating another closure within a short time frame.
- Once below the regulatory limit, toxin levels are decreasing or static in the required number of consecutive samples (dependent on the biotoxin type) in order for the area to be re-opened.
- Other conditions or limitations may be applied if considered necessary by the designated Harvest Area Cordinator and imposed by PrimeSafe.
- The edible portion of each molluscan species harvested from the closed harvesting area meets the following criteria:
9.5 Mechanisms for Re-opening
The Harvest Area Cordinator will reopen a shellfish Harvesting Area to harvesting and movement of shellfish only when each of the reopening criteria have been met.
The Harvest Area Cordinator shall, on each reopening event, prepare documents including the data, environmental conditions and factors leading to that decision.
Resumption of harvesting may be accompanied by increased monitoring where there is a risk of a secondary bloom or low tissue biotoxin levels (less than the regulatory limit) persist.
When harvesting is recommenced in a Harvesting Area, a reopening notice will be issued by the harvest area manager and communicated (fax, post, e-mail or phone) to the following:
- All PrimeSafe licence holders that participate in the shellfish harvest monitoring program within the relevant Harvesting Area(s).
- PrimeSafe.
9.6 Surveillance of harvesting during a biotoxin closure
Surveillance of harvesting during a biotoxin closure is the responsibility of PrimeSafe under the Seafood Safety Act, 2003.
10 Investigation of Illness Due to Toxic Shellfish Poisoning
10.1 Notification
Unlike food or water borne pathogens, suspected cases of toxic shellfish poisoning (TSP) are not notifiable.
10.2 Investigation
Where there is evidence that TSPs are the cause of an illness, it is the responsibility of the DHHSto investigate potential sources of contamination/illness.
Toxic shellfish poisoning investigations should be undertaken in a timely manner and using sound epidemiological principles. This will ensure that valuable information is gained so that TSP events in Australia may be better understood. As is the case with any epidemiological investigation the aim is the control and prevention of further TSP episodes.
All suspected cases of TSP should be investigated. The investigation should include the following foundation steps (not necessarily in the order below):
- Verification of the diagnosis of reported cases and the identification of the specific etiological agent responsible.
- Confirm that an incident exists. Check for other cases at appropriate points such as medical practices in the relevant area.
- Describe the cases in the epidemic or outbreak according to the variables of time, place and person.
- Identify the source of the agent and its mode of transmission, including the specific vehicles, vectors and routes that may have been involved.
- Identify the populations that are at an increased risk of exposure to the agent.
- Plan and implement control measures such as harvesting suspension, the issue of warnings and the implementation of recalls.
- Evaluate the control measures.
Case definitions provide a detailed description of the effects of the various TSP syndromes and are presented in Appendix 8.
10.3 Immediate Action for Suspected Toxic Shellfish Poisoning Cases
10.3.1 Closures of commercial Harvesting Areas
Where investigation indicates that toxic shellfish from PPB or WP shellfish Harvesting Areas have been the cause of illness, an immediate closure will be placed on all of the relevant Harvesting Areas.
Knowledge that the victims had consumed shellfish harvested from one or more of these areas and were suffering symptoms consistent with those from TSP, together with the presence of toxic phytoplankton species above threshold abundance trigger levels or the presence of biotoxins in shellfish tissue, would constitute evidence indicating that the consumption of contaminated shellfish may be the cause of the incident.
Public warnings should be issued pending the results of more detailed investigations. The Public Health Division, DHHS, should issue these in collaboration with PrimeSafe.
10.3.2 Control of movement of harvested shellfish
It is the responsibility of PrimeSafe to undertake a product recall/detention where appropriate as detailed in Section 11, with the cooperation of the appropriate responsible agencies including:
- Office of the Chief Health Officer, Public Health Division, DHHS (Victoria).
- All PrimeSafe licence holders in the relevant harvesting area(s).
10.3.3 Notification
Notices shall be placed in prominent places near Harvesting Areas advising the public of the closure and to advise against consuming shellfish purchased from harvesters in the area between the dates indicated. This notification will be undertaken by the PrimeSafe in consultation with Food Safety Victoria, Public Health Division, DHHS (Victoria).
10.3.4 Communication
Liaison between all appropriate organisations and individuals will be established to ensure that investigations are well co-ordinated. The organisations and individuals may include:
- Office of the Chief Health Officer, Public Health Division, DHHS (Victoria).
- Food Safety Victoria, Public Health Division, DHHS (Victoria).
- PrimeSafe.
- All PrimeSafe licence holders in the relevant harvesting area(s).
- Victorian Marine Farmers Inc.
10.3.5 Sampling
A suite of shellfish tissue sampling may be necessary to facilitate the investigation of a suspected TSP incident.
- Shellfish tissue samples should be taken where available along the distribution pathway from the harvesting area to the suspected TSP sufferer. These may include remains of meals, samples of commercial product from the same batches of product as consumed and samples taken from the suspected harvesting areas.
- Biotoxin levels in shellfish from each harvesting area will be available through the biotoxin monitoring program. Additional sampling and analysis can be performed as required.
- These samples need to be of sufficient size to allow analysis for non-marine biotoxin sources of illness (such as bacterial, viral or chemical contamination) so that these sources can be eliminated as the primary cause of the suspected TSP incident.
- If microbiological testing is required, the sample shall be transported in such a way as to prevent contamination, and identified/labelled appropriately.
- For cases showing gastro-intestinal symptoms, faecal samples should be requested to eliminate bacterial/viral causes of illness.
10.3.6 Funding
Investigation of toxic shellfish poisoning incidents and the associated sampling and testing is funded by the investigating authority/agency.
11 Product Control
11.1 Product Recall
When Harvesting Areas are closed due to the presence of marine biotoxins, and potentially contaminated shellfish have been harvested prior to closure, product willneed to be recalled or detained. However, phytoplankton sampling will usually provide advance warning of any potential risk of shellfish biotoxin poisoning, allowing harvesting restrictions to be implemented before potentially contaminated shellfish are harvested. The recall will include any product harvested since the last satisfactory biotoxin and or phytoplankton sampling event, and should be initiated within 24 hours of Harvest Area closure.
A food product recall is carried out to protect public health and safety. A food withdrawal may also occur as a precautionary measure prior to an official recall or for quality or similar reasons (FSANZ, 2005).
11.2 Objectives
The Food Industry Recall Protocol – A Guide to Writing A Food Recall Plan and Conducting a Food Recall (FSANZ, 2004) notes that there are three primary objectives in any food recall:
- Stop the distribution and sale of an affected product.
- Inform the statutory authorities (all recalls) and the public (consumer recalls only) of the problem.
- Effectively and efficiently remove from the marketplace any product that is potentially unsafe.
11.3 Responsibilities
Product detention and recall will be instigated by PrimeSafe under the Seafood Safety Act, 2003. This process details the recall processes, consumer notification, product detainment and disposal. Food Safety, Department of Health and Human Services also has the power to instigate product detention and seizure, in accordance with the current Food Industry Recall Protocol (FSANZ, 2005).
Product recall is the responsibility of the harvesters, manufacturers, processors, distributors and retailers of affected product, in conjunction with regulators.
Clause 12 of the Food Safety Standard 3.2.2 notes that:
A food business engaged in the wholesale supply, manufacture of importation of food must:
- have in place a system to ensure the recall of unsafe food;
- set out this system in a written document and make this document available to an authorised officer on request; and
- comply with this system when recalling unsafe food.
PrimeSafe licence holders must also prepare food recall plans in accordance with Food Industry Recall Protocol (FSANZ, 2004), again permitting efficient and effective product recall.
11.4 Notification
Notification of food recalls is the responsibility of the business concerned. Guidance can be provided by PrimeSafe, FSANZ or DHHS during the notification process.
Notification should include statutory authorities, PrimeSafe licence holders in the relevant Harvesting Area(s), the product distribution network, Victorian Marine Farmers Inc. and the public (should potentially contaminated product reach the community).
12 References
Arnott, G.H., Reilly, D.J. and Werner, G.F. (1999). Victorian Shellfish Quality Assurance Program. 7. Sanitary Survey Update: Clifton Springs and Grassy Point Portarlington) Aquaculture Zones. Marine and Freshwater Resources Institute, Report No. 13, 1 - 36
ASQAAC (2006). Australian Shellfish Quality Assurance Program Operations Manual
Barry, J.M. and Bailey, M. (2001). Bathymetric Survey of the Proposed Aquaculture Zone, Pinnace Channel Port Phillip. Marine and Freshwater Resources Institute, Report 35: 1-15
Coleman, N., Longmore, A. and Cohen, B. (2001). Baseline Data for the Pinnace Channel Aquaculture Site. Marine and Freshwater Resources Institute, Report 34: 1-35
Commonwealth of Australia (1992). Export Control (Processed Food) Orders. No. 9 of 1992
Commission of European Communities (2001). Draft Commission Decision of Establishing the Methods of Analysis and the Maximum Limits for Certain Marine Biotoxins in Bivalve Molluscs, Echinoderms, Tunicates and Marine Gastropods. Brussels, SANCO/2227/2001 Rev 3
Department of Primary Industries (2005). Eastern Port Phillip Bay Aquaculture Fisheries Reserve Management Plan. Fisheries Victoria Management Series 33: 1-53, Appendices 1-13
Department of Primary Industries (2005). Flinders Aquaculture Fisheries Reserve Management Plan. Fisheries Victoria Management Series 32: 1-51, Appendices 1-12
Department of Primary Industries (2005). Geelong Arm Aquaculture Fisheries Reserve Management Plan. Fisheries Victoria Management Series 34: 1-56, Appendices 1-15
Department of Primary Industries (2005). Pinnace Channel Aquaculture Fisheries Reserve Management Plan. Fisheries Victoria Management Series 31: 1-48, Appendices 1-9
Ecowise Environmental (2008). Victorian Shellfish Quality Assurancee Program. Victorian Marine Biotoxin Management Plan. Edition 2. Prepared for Fisheries Victoria by Ecowise Environmental.
Fisheries Victoria (2003). Pinnace Channel Aquaculture Fisheries Reserves – Current, Wind and Wave Data. Fisheries Victoria Research Report Series, 2: 1-15
Fisheries Victoria (2004a). Geelong Arm Aquaculture Fisheries Reserves – Current, Wind and Wave Data. Fisheries Victoria Research Report Series, 4: 1-19
Fisheries Victoria (2004b). Environmental Characterisation of the Aquaculture Fisheries Reserves in the Geelong Arm, Port Phillip Bay, Victoria. Fisheries Victoria Research Report Series, 8: 1-45
Fisheries Victoria (2004c). Environmental Characterisation of the Aquaculture Fisheries Reserves in Eastern Port Phillip Bay, Victoria. Fisheries Victoria Research Report Series, 10: 1-39
Fisheries Victoria (2004d). Eastern Port Phillip Bay Aquaculture Fisheries Reserves – Current, Wind and Wave Data. Fisheries Victoria Research Report Series, 17: 1-17
FSANZ (2005). Food Standards Code. Standard 4.2.1 Primary Production and Processing Standard for Seafood. Food Standards Australia and New Zealand
FSANZ (2004). Food Industry Recall Protocol. A Guide to Writing a Food Recall Plan and Conducting a Food recall. 5thEdition. Food Standards Australia New Zealand
Hallegraeff, G. (1997). Algal toxins in Australian Shellfish. In: Foodborne Microorganisms of Public Health Significance. Fifth Edition. AIFST (NSW Branch), Food Microbiology Group
Hallegraeff, G. (2002). Aquaculturists' Guide to Harmful Australian Microalgae. The Print Centre, Hobart.
Holland, P.T., McNabb, P., Selwood, A., Page, T., Bell, K and MacKenzie, L. (In Press). Marine Biotoxin Monitoring of New Zealand Shellfish – A New Management Programme Based on LC-MS. In: Proc. 2nd Int. Conference on Harmful Algae Management and Mitigation. Nov 2001, Qingdao, China. Hall, S. & Zou, YL (Ed.)
MacKenzie, L. (2002). An Evaluation of the Risk to Consumers of Pectenotoxin 2 seco acid (PTX2-SA) Contamination of GreenshellÔ Mussels. Prepared for Marlborough Sounds Shellfish Quality Programme. Cawthron Institute Report 750, 1- 50
MacKenzie, L., Holland, P., McNabb, P., Beuzenberg, V., Selwood, A. and Suzuki, T. (2002). Complex Toxin Profiles in Phytoplankton and Greenshell Mussels (Perna canaliculus), Revealed by LC-MS/MS Analysis. Toxicon, 40: 1,321 – 1,330
McKinnon, L.J., Leporati, S.C., Parry, G.D. and Blake, S. (2004). Environmental Characterisation of the Flinders Aquaculture Reserve in Western Port, Victoria. Fisheries Victoria Research Report Series, 14: 1 - 18
Todd, K. (2001). Australian Victorian Marine Biotoxin Management Plan for Shellfish Farming. Prepared for the Australian Shellfish Quality Assurance Committee (ASQAAC) by the Cawthron Institute. Cawthron Report No. 645
Todd, K. (2002). A Review of NSP Monitoring in New Zealand In Support of a New Programme. Prepared for Marine Biotoxin Technical Committee. Cawthron Report No. 660, 1 - 30
*WATER ECOscience (2002). WATER ECOscience VSQAP Operations Manual
(Now Ecowise Environmental)
APPENDICES
Appendix 1 – Agency and Personnel Contacts
Agency / Contact | Responsibility | Contact Details |
|---|---|---|
PrimeSafe | Authority | PO Box 2057, South Melbourne, VIC 3205
|
Andrew Coghill (1) | Manager, Compliance and Enforcement Services | 03 9685 7377 |
Amita Bernadi | Manager, Information and Support Services | 03 9685 7399 |
Office of Chief Health Officer, DHHS
|
| Department of
Health and Human Services |
Dr Rosemary Lester | Victoria's Chief Health Officer | 03 9096 0376 |
Department of Health and Human Services |
| Department of
Health and Human Services
|
Fiona Jones | Manager Regulation & Incident Management | 03 9096 5098 |
EPA Victoria | Marine Science - Environmental monitoring & policy | Centre for Environmental Sciences |
Victorian Marine Farmers (VMF) | Industry body | Victorian Marine Farmers (VMF)
|
Michael Houghton | President | 0412 491 977 |
Appendix 2 – Laboratories and Contacts for Phytoplankton Enumeration & Identification
Agency / Contact | Capability/Position | Contact Details |
|---|---|---|
Microalagal Services | Phytoplankton Identification and Enumeration
| 308 Tucker Road |
Dr Stephen Brett (1) | Senior Botanist | (03) 9578 2158 |
Dr David Hill (2) | Senior Botanist | (03) 9578 2158 |
Adele Neale (3) | Biological Analyst | (03) 9578 2158 |
University of Tasmania Institute for Marine and Antarctic Studies (IMAS) | Phytoplankton Identification, Electron- microscopy, Phytoplankton Culture, DNA Probes | Private Bag 5129, Hobart, TAS, 7001
|
Prof Gustaaf Hallegraeff | Phytoplankton Taxonomy Electron Microscopy | 03 6226 2623 |
(1) Primary Contact (2) Secondary Contact (E) Emergency Contact
Appendix 3 – Approved Laboratories and Contacts for Marine Biotoxin Analysis of Shellfish Flesh
Agency / Contact | Responsibility/Position | Contact Details |
|---|---|---|
Advanced Analytical Australia | PST (LC-FLD), DST, AST, NST (LCMSMS) (NATA Accreditation No. 15109) | 11 Julius Avenue,
North Ryde NSW 2113 |
Andrew Bradbury (1) | National Business Development Manager | 07 3268 1228 |
Ross Briety (2) | State Manager Victoria | 03 9376 1999 |
Ryan Tombs (3) | Team Leader customer service | 02 9888 9077 |
Medvet Sciences, IMVS Food & Environmental Laboratory
| NST Screen (Mouse Bioassay) (NATA Accreditation No. 1521) | PO Box 14, Rundle
Mall, SA 5000 |
Fil Lagala (1) | Laboratory Manager | 08 8222 3363 |
Peter Cameron (2) | Laboratory Supervisor | 08 8222 3363 |
Queensland Health Scientific Services | AZA, YTX Analysis (Qualitative) (HPLC Electrospray MS) | PO Box 594,
Archerfield, QLD 4108 |
Dr Geoff Eaglesham (1) | Supervising Scientist | 07 3274 9085 |
Steve Carter (2) | Scientist | 07 3274 9085 |
Cawthron Institute Biotoxin Laboratory | DSP Analysis (LC-MS/MS) NSP Analyses (LC-MS/MS, Mouse Bioassay) | Private Bag 2 Nelson, New Zealand |
Catherine Moisan | Technical Manager | 0011 643 548 2319 |
(1) Primary Contact (2) Secondary Contact (E) Emergency Contact
Appendix 4 – Sampling Officers
All sampling officers must have undergone training provided by Fisheries Victoria or be deemed by Fisheries Victoria to have sufficient experience in the sampling of shellfish for bacterialogical, chemical and biotoxin sampling, and water sampling for bacteria and microalgae. Fisheries Victoria will make available training as required and will maintain a list of approved samplers that records the samplers name, harvesting area and contact details.
Appendix 5 – Marine Biotoxin Analytical Methods
Advanced Analytical Australia is contracted to provide PSP, ASP, DSP services (since July 2012).
Toxin | Method | Lower Limit of Reporting |
PSP group STX, GTX1,4, Neo, GTX2,3, dcSTX, dcNeo, dcGTX2,3, C1,2, C3,4, GTX5 | PSP screening by LC-FLD (Lawrence Method)* | 0.025 mg/Kg |
PSP confirmation by LC-FLD AOAC 2005.06 (Lawrence Method)* | 0.025 mg/Kg | |
DSP group AZA1, AZA2, AZA3, total DTX1, free DTX1, total DTX2, free DTX2, Total OA, free OA, Gymnodimine, PTX2, Spirolide, | LCMSMS (McNabb, P., Selwood, A.I., Holland, P.T. (2005). J. AOAC Int. 88(3), 761-772.) | 0.025 mg/Kg |
ASP Domoic Acid | LCMSMS (McNabb, P., Selwood, A.I., Holland, P.T. (2005). J. OAC Int. 88(3), 761-772.) | 0.025 mg/Kg |
*For the PSP group of toxins the laboratory is able to carry out a rapid screening test whereby the analysis does not separate all the various toxins belonging to the PSP group but detects some of them as a group. Individual toxins within this group have differing toxicities but for the purpose of a rapid assay all members of the group are assumed to be as toxic as the most toxic member of the group and the level of toxin in the sample calculated on that basis. The total toxin level so determined is therefore likely to be an over estimation of the actual toxin level so in the event that the 'screen" level exceeds FSANZ standards a second assay (PSP confirmation assay) which separates and analyses separately the various members of the group may be necessary.
Other services available include:
- IMVS screen samples for NSP toxins using mouse bioassay.
- Cawthron Institute in New Zealand can conduct all toxicity tests, using the chemical confirmatory and screening test methods.
Appendix 6 – Phytoplankton Sampling Procedures
Collecting phytoplankton samples using the hosepipe sampler
Purpose:
As noted in the VSOM, the aim is to collect a depth integrated sample of phytoplankton for enumeration over the entire depth of the mussel lines appropriate to industry practice. This is preferred over a surface sample due to variability in the vertical distribution of phytoplankton. The sample collected will be used to enumerate any toxic phytoplankton present.
Equipment:
- 25 mm internal diameter hosepipe sampler of appropriate length (marked at 1m intervals and weighted at bottom end).
- Strong line attached to bottom of sampler at the weight.
- Spare bungs for hosepipe sampler.
- Clean bucket (>12L volume).
- 1L sample bottles - 1 for each sample taken plus spares.
- Labels (and Lugol's preservatives if required).
- Eskies for transporting samples.
Care is to be taken that ALL equipment is attached securely to the boat.
Method:
Prepare hosepipe sampler
- Make sure top end is firmly attached to the boat.
- Ensure bottom line is attached firmly both to the bottom of the hosepipe and the other end to the boat.
- Remove bung from top end (or open tap or valve if present).
Collect sample
- Lower weighted, bottom end very slowly to appropriate depth, to avoid disturbing any layers of phytoplankton in the water column.
- Take care not to hit the bottom, particularly at low tide and/or where there is a swell.
- If the bottom is hit, discard the sample, clean the sampler and re-take the sample at a lesser depth.
Retrieve sample
- Replace bung securely in top of tube and pull the bottle and the sample up into the boat; make sure the bung remains firmly in place (refer diagram).
- Insert the bottom end of hosepipe into the bucket, remove the bung and empty sample into the bucket.
Fill sample bottles
- Label sample bottle(s) with time and date of sampling, sample type and harvesting area.
- Gently mix sample in bucket.
- Sub-sample by lowering a plastic, labelled 1L bottle into bucket and fill, leaving a 10 cm air space at top; cap bottle firmly.
- Fill required number of plastic bottles with sample water.
- Store samples in an esky with 1 icepack to keep cool (icepack NOT in contact with samples– purpose is merely to keep esky cool while samples are transported to the laboratory).
- Generally samples are returned to the laboratory live but some can be preserved with Lugol's iodine or other preservatives in the field. If samples are preserved, note on the label clearly.
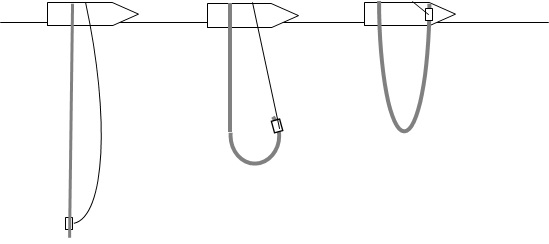
Collecting phytoplankton using the plankton net
Purpose:
To collect a concentrated phytoplankton sample along the entire depth of the mussel lines for the purposes of detecting and identifying nuisance species, including those that may be present in low numbers.
Equipment:
- 20mm mesh plankton net of 300mm diameter and 1 metre length.
- 75 mL polycarbonate net bottles fitting the plankton net end.
- 125 mL plastic sample storage vials.
- Labels.
- Lugol's iodine or other preservative if required.
- Weight to attach to plankton net to facilitate sinking.
Method:
Check equipment
- Ensure net line is firmly attached both to the net and the boat.
- Wash net and bottle prior to use.
- Screw or otherwise attach plastic bottle onto net.
- Label and prepare 125 mL sample storage vials.
Collect sample
- Lower net to an appropriate depth.
- Do not allow the net weight to hit the bottom (clean net and repeat sample if it does).
- Slowly but steadily pull the net up to the boat.
- Wash material adhering to inside of net towards the net bottle end by gently dipping and shaking the net.
Fill and store sample bottles
- Carefully remove 75mL bottle from the net end.
- Transfer sample to the appropriate, labelled 125mL storage vials leaving a 10 - 20mm air space.
- Cap tightly and store with other algal samples in an esky with a single ice pack (ice pack NOT in contact with samples – purpose is merely to keep esky cool while samples are transported to the laboratory).
- If further samples are required, wash net and repeat as above.
- Generally samples are returned to the laboratory live but some can be preserved with Lugol's iodine or other preservatives in the field. If samples are preserved, note on the label clearly.
- Wash the plankton net and net bottle prior to leaving the site or taking additional samples.
Appendix 7 – Phytoplankton Species Lists
The following lists are presented to summarise the phytoplankton species that potentially produce biotoxins and present a potential risk of human illness resulting from the consumption of shellfish contaminated with these toxins. It is stressed that the tables are "all inclusive" and that there is great variability in the level of evidence resulting in the inclusion of species as potentially toxic. This evidence varies from that which is circumstantial at best (e.g. species was present during a single incident at one locality which had several potential causes, one of which was biotoxins) to very powerful evidence of widespread toxicity supported by detailed biotoxin studies. The tables are presented as a guide and it is crucial that they be modified to incorporate local and international information as it comes to hand and that management decisions are made with full awareness of why a species was listed or unlisted as potentially toxic.
Nonetheless, all records of toxicity should be examined carefully as the toxicity of specific algal species may vary substantially between different geographical areas and even from time to time in the same area. In addition, there are records of the introduction of new forms in recent years through agents such as ballast water e.g. Gymnodinium catenatum into South East Tasmanian waters. The potential therefore exists for the introduction of toxic species or strains not seen in an area previously.
Categories A2 – C are essentially reproduced from the Australian Victorian Marine Biotoxin Management Plan for Shellfish Farming (2001). In addition, detailed records of phytoplankton occurrence and biotoxin presence in shellfish have been collected as part of the Victorian Shellfish Quality Assurance Program extending back to 1987. This together with other information presented in the literature has permitted the presentation of an additional list of potentially toxic phytoplankton specific to the regions of Port Phillip Bay and Western Port containing shellfish harvest areas. This is presented as Category A1 and is modified from Category A as presented in the Australian Victorian Marine Biotoxin Management Plan for Shellfish Farming (2001). It is stressed that this list should only be used in relation to those harvest areas covered by the Victorian Marine Biotoxin Management Plan to date, Port Phillip Bay and Western Port. Phytoplankton listed as Category A1 may also be included in the Category A2 list.
Category A1: Species occurring in south-eastern Australian waters, which are known or suspected toxin producers in Australia.
Species | Toxins/Comments |
Bacillariophyceae (Diatoms) | |
Pseudo-nitzschia australis | ASP (domoic acid) |
Pseudo-nitzschia multiseries | ASP (domoic acid) |
Pseudo-nitzschia delicatissima
Pseudo-nitzschia galaxiae | NT in PPB & Tas ASP (domoic acid) overseas NT in all PPB isolates so far |
Pseudo-nitzschia pungens | NT in PPB and Bass Strait Toxic strains elsewhere incl. NZ - ASP (domoic acid) |
Pseudo-nitzschia pseudodelicatissima | NT in PPB, Vic, NSW One of main bloom species in PPB, Vic and Tas Toxic strains elsewhere? - ASP (domoic acid) |
Pseudo-nitzschia multistriata | NT in Aust? Very common. ASP (domoic acid) New Zealand (weakly toxic) |
Dinophyceae (dinoflagellates) | |
Alexandrium catenella | PSP (Saxitoxins, C1 - C4, gonyautoxins) |
Alexandrium tamarense | NT in all Australian isolates so far – some toxic strains? PSP (Saxitoxins, C1 - C4, gonyautoxins) |
Alexandrium fundyense | A. fundyense from PPB shown to be A. catenella |
Alexandrium minutum | PSP (Saxitoxins, mostly gonyautoxins) |
Alexandrium ostenfeldii
Alexandrium insuetum | Not linked to toxicity in Aust; non-bloom forming Sometimes toxic NZ – saxitoxins and derivatives Canada - spirolides NT in all PPB isolates so far |
Dinophysis acuminata | DSP - Weakly toxic in NZ OA , ?DTX 3 (not tested yet) |
Dinophysis caudata | ?DSP (?OA, ?DTX 1 – 3) |
Dinophysis fortii | ?DSP (?OA, ?DTX 1 – 3) |
Dinophysis acuta | ?DSP (?OA, ?DTX 1 – 3); DSP in NZ |
Dinophysis miles | ?DSP (?OA, ?DTX 1 – 3) |
Dinophysis tripos | ?DSP (?OA, ?DTX 1 – 3) |
Prorocentrum lima | ?DSP (?OA, ?DTX 1 – 3) |
Gymnodinium catenatum | PSP (sulphamate saxitoxins) |
Karenia cf brevis | ?NSP (BTX) |
Karenia mikimotoi | ?NSP – NR of toxicity in Aust to date Non BTX producer in NZ; Gymnocin in Japan |
NT = Non Toxic PPB = Port Phillip Bay Vic = Victoria Tas = Tasmania NZ = New Zealand Aust = Australia
OA = Okadaic acid DTX = Dinophysis toxins DTX3 = diol esters BTX = brevetoxins
? Indicates this toxin has not been confirmed in Australian strains of this species, at the time of this report.
Category A2: Species known to be present in south-eastern Australian waters and proven to produce toxins either in Australia or internationally. (Modified from Australian Victorian Marine Biotoxin Management Plan for Shellfish Farming (2001)
Species | Toxins/Comments |
Pseudo-nitzschia australis | ASP (domoic acid) |
Pseudo-nitzschia delicatissima | ASP (domoic acid) |
Pseudo-nitzschia fraudulenta | ASP (domoic acid); NT Australia, weakly toxic NZ |
Pseudo-nitzschia multiseries | ASP (domoic acid) |
Pseudo-nitzschia pseudodelicatissima | ASP (domoic acid) |
Pseudo-nitzschia pungens | ASP (domoic acid) Usually NT but some strains produce high ASP levels |
| ASP (domoic acid) |
Pseudo-nitzschia turgidula | ASP (domoic acid); NT Australia, weakly toxic NZ |
Alexandrium catenella | PSP (saxitoxin and derivatives) |
Alexandrium minutum | PSP (saxitoxin and derivatives) |
Alexandrium ostenfeldii | PSP (saxitoxin and derivatives) Spirolides in Canada |
Alexandrium tamarense | PSP (saxitoxin and derivatives) Also has non-toxic strains |
Dinophysis acuminata | DSP (OA?, DTX 1 – 3?) |
Dinophysis acuta | DSP (OA?, DTX 1 – 3?) |
Dinophysis caudata | DSP (OA?, DTX 1 – 3?) |
Dinophysis fortii | DSP (OA?, DTX 1 – 3?) |
Dinophysis hastata | DSP (OA?, DTX 1 – 3?) |
Dinophysis mitra | DSP (OA?, DTX 1 – 3?) |
Dinophysis rotundata | DSP (OA?, DTX 1 – 3?) |
Dinophysis tripos | (DSP (OA?, DTX 1 – 3?) Some strains only |
Gymnodinium catenatum | PSP (saxitoxin and derivatives) |
Karenia cf brevis | NSP (brevetoxins) |
Gymnodinium aureolum | ?NSP (?weakly toxic in NZ) |
Karlodinium micrum | ?NSP (?weakly toxic in NZ); fish killer |
Prorocentrum lima | DSP (OA?, DTX 1 – 3?) |
Pyrodinium bahamense var. compressum | Tropical habitats PSP (saxitoxin and derivatives) |
NT = Non Toxic ? DTX 3 = OA esters Indicates this toxin has not been confirmed in Australian strains of this species, at the time of this report .
Category B: Potential toxin producing species (i.e.toxicity untested/unclear) known to be present in Australian coastal waters including species known/suspected to be toxic overseas (Modified from Australian Victorian Marine Biotoxin Management Plan for Shellfish Farming (2001).
Species | Toxins/Comments |
Azadinium spp. | Possibly AZA1-3 Toxic in New Zealand |
Pseudo-nitzschia cuspidata | Possibly ASP (domoic acid) |
Pseudo-nitzschia heimii | Possibly ASP (domoic acid) Non-toxic in New Zealand; toxicity unknown elsewhere |
Pseudo-nitzschia lineola | Possibly ASP (domoic acid) |
Pseudo-nitzschia multistriata | Possibly ASP (domoic acid) Non-toxic in New Zealand |
Pseudo-nitzschia subfraudulenta
| Possibly ASP (domoic acid) |
Pseudo-nitzschia subpacifica | Possibly ASP (domoic acid) |
Alexandrium pseudogonyaulax | Possibly PSP (STX and derivatives, goniodomin) |
Chattonella marina/antiqu | Possibly NSP (brevetoxins) |
Fibrocapsa japonica | Possibly NSP (brevetoxins) |
Heterosigma akashiwo | Possibly NSP (brevetoxins) |
NT = Non Toxic STX = saxitoxin
Category C: Other potential toxin producing species world-wide that may be present in Australian waters (Modified from Australian Victorian Marine Biotoxin Management Plan for Shellfish Farming (2001).
Species | Toxins/Comments |
Alexandrium angustitabulatum | Possibly PSP (saxitoxin and derivatives) Present in New Zealand |
Alexandrium acatenella | Possibly PSP (saxitoxin and derivatives) |
Alexandrium cohorticula | Possibly PSP (saxitoxin and derivatives) |
Alexandrium fraterculus | Possibly PSP (saxitoxin and derivatives) |
Alexandrium fundyense | Possibly PSP (saxitoxin and derivatives) |
Alexandrium lusitanicum | Possibly PSP (saxitoxin and derivatives) |
Alexandrium tamiyavanichi | Possibly PSP (saxitoxin and derivatives) |
Coolia monotis | Cooliatoxin |
Dinophysis norvegica | Major DSP producer in Europe |
Gymnodinium aureolum | Possibly NSP (brevetoxins) Low levels of BTX in New Zealand; NT in Aust? |
Gymnodinium impudicum | Possibly NSP (brevetoxins) Low levels of BTX in New Zealand? |
Gymnodinium pulchellum | Possibly NSP (brevetoxins) NT in PPB and Aust? |
Karenia bidigitata | Possibly NSP (brevetoxins) Low levels of BTX in New Zealand? |
Karenia brevisulcata | Wellington Harbour Toxin (WHT) Low levels of BTX in New Zealand? |
Karenia papilionacea | Possibly NSP (brevetoxins) |
Karenia selliformis | Gymnodimine and low BTX levels - New Zealand |
Karlodinium micrum | Possibly NSP (brevetoxins) – low BTX levels in NZ |
Lingulodinium polyedra | Yessotoxins in Japan |
Nitzschia navis-varingica | ASP(domoic acid) in brackish Vietnamese waters |
Ostreopsis siamensis | Ostreotocin |
Pfiesteria piscicida | Toxin being characterised |
Prorocentrum concavum | DSP (OA?, DTX 1 – 3?) |
Prorocentrum elegans | DSP (OA?, DTX 1 – 3?) |
Prorocentrum hoffmannianum | DSP (OA?, DTX 1 – 3?) |
Prorocentrum maculosum | Prorocentrolides |
Prorocentrum minimum | The toxin linked to this organism (185 fatalities in Japan) has not yet been elucidated, and the role of P. minimum is still in question |
Protoceratium reticulatum | Yessotoxin producer in New Zealand |
NT = Non Toxic PPB = Port Phillip Bay BTX = brevetoxin DTX 3 = OA esters
? Indicates this toxin has not been confirmed in Australian strains of this species, at the time of this report .
Category D:Nuisance species known to be present in Australian waters that are not known to be toxic to humans but are to be monitored for other reasons including potential for economic damage to industry and its reputation (Modified from Australian Victorian Marine Biotoxin Management Plan for Shellfish Farming (2001).
Species | Toxins/Comments |
Rhizosolenia cf chunii | NT but produces a bitter taste in mussels, oysters and scallops in PPB. |
Appendix 8 – Toxic Shellfish Poisoning Case Definitions
Surveillance Case Definition for all Forms of Toxic Shellfish Poisoning
Suspected case (general clinical case definition)
- Vomiting or diarrhoea occurring within 24 hours of consuming shellfish
- Any of the following neurological symptoms occurring within 24 hours of consuming shellfish:
- Neurosensory symptoms
- Paraesthesia, i.e. numbness or tingling around the mouth, face or extremities
- Alternation of temperature sensations such as a prickly feeling on the skin during a bath/shower or exposure to sun, or difficulty distinguishing hot or cold objects
- Neuromotor/neurocerebellar symptoms
- Weakness such as trouble rising from seat or bed
- Difficulty swallowing
- Difficulty breathing
- Paralysis
- Clumsiness
- Unsteady walking
- Dizziness/vertigo
- Slurred/unclear speech
- Double vision
- Neurosensory symptoms
- One or more of the following neurological signs/symptoms occurring within 48 hours of consuming shellfish:
- Confusion
- Memory loss
- Disorientation
- Seizure
- Coma
Paralytic Shellfish Poisoning (PSP) Case Definition
Suspected case (clinical case definition)
The following neurological symptoms occurring within 12 hours of consuming shellfish:
- Neurosensory paraesthesia i.e. numbness or tingling around the mouth, face or extremities
- And one of the following neuromotor/neuro-cerebellar symptoms:
- Weakness such as trouble rising from seat or bed
- Difficulty in swallowing
- Difficulty in breathing
- Paralysis
- Clumsiness
- Unsteady walking
- Dizziness/vertigo
- Slurred/unclear speech
- Double vision
- Meets the case definition
- And within 7 days of the collection of shellfish consumed by the case, PSP biotoxins are detected at or above the regulatory limit (currently 80 µg/100 g tissue) in shellfish obtained from near or at the same site (not leftovers).
- Meets the clinical case definition
- AND PSP biotoxins are detected in leftover shellfish at a level that meant the case consumed a dose likely to cause illness (current level: 10 MU/kg body weight, about 2 µg/kg body weight).
Probable case
Confirmed case
Amnesic Shellfish Poisoning (ASP) Case Definition
Suspected case (clinical case definition)
- Vomiting or diarrhoea or abdominal cramps, occurring within 24 hours of consuming shellfish
- And no other probable cause identified by microbiological examination of a faecal specimen from the case or microbiological testing of left-over food
- And/or one or more of the following neurological signs/symptoms occurring within 48 hours of the consumption of the shellfish:
- Confusion
- Memory loss
- Disorientation
- Seizure
- Coma
- Meets the clinical case definition
- And within 7 days of the collection of shellfish consumed by the case ASP biotoxins are detected at or above the regulatory limit (currently 20 ppm domoic acid/100 g tissue) in shellfish obtained from near or at the same site (not leftovers).
- Meets the clinical case definition
- And ASP biotoxins detected in leftover shellfish at a level resulting in the case consuming a dose likely to cause illness (current level: 0.05 mg/kg body weight).
Probable case
Confirmed case
Diarrhetic Shellfish Poisoning (DSP) Case Definition
Suspected case (clinical case definition)
- Vomiting or diarrhoea occurring within 24 hours of consuming shellfish
- And no other probable cause identified by microbiological examination of a faecal specimen from the case or microbiological testing of left-over food.
- Meets the clinical case definition
- And within 7 days of collection of shellfish consumed by the case, DSP biotoxins are detected at or above the regulatory limit (currently 20 µg/100 g shellfish or 5 MU/100 g) in shellfish obtained from near or at the same site (not leftovers).
- Meets the clinical case definition
- And detection of DSP biotoxins in leftover shellfish at a level resulting in the case consuming a dose likely to cause illness (current level: ingestion of 48 µg or 12 MU).
Probable case
Confirmed case
Neurotoxic Shellfish Poisoning (NSP) Case Definition
Suspected case (clinical case definition)
Two or more of the following neurological symptoms occurring within 24 hours of consuming shellfish:
- Neurosensory:
- Paraesthesia i.e. numbness or tingling around the mouth, face or extremities
- Alternation of temperature sensations such as a prickly feeling on the skin during a bath/shower or exposure to sun, or difficulty distinguishing hot or cold objects
- Neuromotor/neurocerebellar:
- Weakness such as trouble rising from seat or bed
- Difficulty in swallowing
- Difficulty in breathing
- Paralysis
- Clumsiness
- Unsteady walking
- Dizziness/vertigo
- Slurred/unclear speech
- Double vision
Probable case
- Meets the clinical case definition
- And within 7 days of collection of shellfish consumed by the case, NSP biotoxins detected at or above the regulatory limit (currently 20 MU/100 g shellfish) in shellfish obtained from near or at the same site (not leftovers).
- Meets the clinical case definition
- Detection of NSP toxins in leftover shellfish at a level resulting in the case consuming a dose likely to cause illness (current level: 0.3 MU/kg body weight).
Confirmed case
Appendix 9 – Phytoplankton Action Levels
The following table summarises the phytoplankton levels (in cells/litre) that are used to trigger the sampling of shellfish flesh for biotoxin analysis and harvesting suspensions. These levels are derived from levels used internationally and in various States in Australia. They have been modified in accordance with specific information obtained pertaining to phytoplankton presence/abundance and biotoxin levels in shellfish tissue as part of shellfish quality assurance monitoring, in Port Phillip Bay and Western Port. They should be further revised as additional monitoring and research is undertaken and supports a change.
Note: For Pseudo-nitzschia spp risk remains high for a minimum of two weeks post bloom crash.
Phytoplankton Abundance Triggers for this Victorian Marine Biotoxin Management Plan (cells/L) | ||||||||
Alga / Algal Group | Toxin | Definitive Identification & Warning to Growers | Tissue Testing | Harvest Suspension Pending Toxin Analysis | Harvest Resumption | |||
Bacillariophyceae | ||||||||
Pseudo-nitzschia spp. (pseudodelicatissima group and pungens)** | ASP (domoic acid) | 100,000 | 300,000 | 500,000 | <20 mg/kg domoic acid for 2 successive samples taken at least 14 days apart; phytoplankton abundance not rising. | |||
Pseudo-nitzschia spp. australis & multiseries (in seriata group) | ASP | 100,000 | 100,000 | 300,000 | As Above | |||
Rhizosolenia cf chunii | Bitter Taste | 10,000 | N/A | 20,000 Level 2 Warning | Harvesting suspended/resumed by growers depending on taste of mussels. | |||
Dinophyceae | ||||||||
Alexandrium catenella | PSP | 200 | 200 | 500 | <0.8 mg/kg PSP for 2 successive samples at least 48 h apart; phytoplankton abundance not rising. | |||
Alexandrium minutum | PSP | 200 | 200 | 500 | As Above | |||
Alexandrium tamarense | PSP Some strains | 200 | 200 | 500 | As Above | |||
Alexandrium spp. (unknown or in doubt) | PSP Some strains | 200 | 200 | 500 | As Above | |||
Azadinium spp. | AZA1-3 | 30,000 | 30,000 | 30,000 | Precautionary limit same as NZ limit | |||
Gymnodinium catenatum | PSP | 1,000 | 1,000 | 5,000 | <0.8 mg/kg PSP for 2 successive samples taken at least 48 h apart; phytoplankton abundance not rising. | |||
*Dinophysis acuminata | DSP | 1,000 | 1,000 | 2,000 | <0.16 mg/kg DSP for 2 successive samples taken at least 48 h apart; phytoplankton abundance not rising. | |||
Dinophysis caudata Dinophysis fortii Dinophysis acuta | DSP | 1,000 500 | 1,000 500 | 2,000 1,000 | As Above | |||
Dinophysis spp. | ?DSP | 500 | 500 | 1,000 | As Above – precautionary only till further information available | |||
Karenia brevis (Not currently recorded in Aust) | NSP brevetoxin (BTX) | 1,000 | 2,000 | 5,000 | < 0.8 BTX-2 eq for 2 successive samples taken at least 48 h apart; phytoplankton abundance not rising. | |||
Karenia mikimotoi, K. papilionacea, K. bidigitata, K. brevisulcata, K. selliformis Flat, Australian species morphologically similar to K. brevis or K. mikimotoi Karlodinium micrum, Gymnodinium impudicum | < 0.8 BTX-2 eq for 2 successive samples taken at least 48 h apart; phytoplankton abundance not rising. | |||||||
| ?NSP | 100,000 | 250,000 | 300,000 | ||||
Prorocentrum lima | ?DSP | 500 | 500 | 1,000 | <0.16 mg/kg DSP for 2 successive samples taken at least 48 h apart; phytoplankton abundance not rising. | |||
* Draft Australian Victorian Marine Biotoxin Management Plan for Shellfish Farming (2001) trigger adopted for now until more information on DTX-3 (OA esters) is available for PPB; PTX2-SA no longer included as toxins.
NOTE: Harvest suspension pending biotoxin analysis is precautionary; suspension / resumption of harvesting will be determined by toxin levels and their regulatory limit as noted below.
**Unless these Pseudonitzschiaspecies are distinguished definitively from the lower toxicity group (which cannot be done with analysis by light microscopy) the lower trigger levels as specified for the P. australis group must be applied.
Appendix 10 – Marine Biotoxin Regulatory and Advisory Levels
The following table shows the maximum levels for each regulated marine biotoxin group as required by the FSANZ Food Standards Code (2005), and the maximum levels applied in the Victorian Marine Biotoxin Management Plan for toxins that are not regulated in the Food Standards Code (yellow shading).
Victorian Marine Biotoxin Management Plan maximum levels by biotoxin group.
Toxin Class | Units | Regulatory Limita | Victorian | Method | Limit of Detection | Laboratory Utilised |
PSP | mg/kg | 0.8 STX eq | 0.8 STX eq | LC-FLD | 0.05 | Advanced Analytical Australia |
ASP (domoic acid) | mg/kg | 20 | 20 | LC-MS/MS | 0.025 | Advanced Analytical Australia |
DSP | mg/kg | 0.2 b | 0.16 b | LC-MS/MS | 0.025 | Advanced Analytical Australia |
NSP | mg/kg MU/kgc | 0.8 BTX-2 eq 200 | 0.8 BTX-2 eq NA | LC-MS/MS Mouse Bioassay | 0.1 – 0.2 10 | Advanced Analytical Australia/Cawthron Institute (NZ) |
Yessotoxins (YTX) | mg/kg | 1.0 | 3.75 | LC-MS/MS | 0.025 | Advanced Analytical Australia |
Azaspiracids (AZA) | mg/kg | 0.16 | 0.16 | LC-MS/MS | 0.025 | Advanced Analytical Australia |
a PSP, ASP, DSP & NSP regulatory limits from FSANZ Food Standards Code (2005). Yessotoxins and azaspiracids are not currently regulated in Australia under the Food Standards Code.
b DSP toxins include okadaic acid, dinophysistoxins (DTX1, DTX2, DTX3) and pectenotoxins. Pectenotoxin-2 seco acid is not included.
c MU = mouse units
Notes on detection and quantification of biotoxin levels:
Toxins regulated under the Food Standards Code:
Paralytic Shellfish Poisoning (PSP)
Analysis is by Liquid chromatography– Fluorescence Detector (LC-FLD). The maximum level is PSP toxins greater than or equal to 0.8 mg of saxitoxin equivalents/kg of edible shellfish flesh determined by the sum of the toxicity equivalent factors (TEFs) for all individual PSP toxins. The Food Standards Code does not specify the toxicity equivalence factors. The Victorian Marine Biotoxin Management Plan utilises the TEFs specified in Oshima (1995)[1]with the exception of the TEF for neo-saxitoxin, for which a TEF of 2.54 is utilised. This is a precautionary measure based on the studies of oral toxicity of Paralytic Shellfish Toxins undertaken by Munday et al. (2013)[2], which showed that the oral toxicity of neo-STX was significantly higher than that previously assessed by intraperitoneal injection.
Amnesic Shellfish Poisoning (ASP)
Analysis is by Liquid chromatography–mass spectrometry/mass spectrometry (LC-MS/MS). The maximum level is greater than or equal to 20 mg/kg of domoic acid and its isomers in the edible shellfish flesh. No toxicity equivalent factors are set for the isomers of domoic acid, which for regulatory purposes are assumed to be equipotent to domoic acid.
Neurotoxic Shellfish Poisoning (NSP)
Historically a maximum level of NSP toxins greater than or equal to 200 mouse units/kg of edible shellfish flesh was applied, using analysis by ether extraction and mouse bioassay with a maximum observation time of 6 hours. Currently analysis of brevetoxin (BTX-1, BTX-2 & BTX-3) levels is undertaken by Liquid chromatography–mass spectrometry/mass spectrometry (LC-MS/MS). The maximum level applied to the results of this analysis is measured in BTX-2 equivalents (i.e. 0.8mg/kg BTX-2 equivalents/kg shellfish), which is considered to be equivalent to 200 mouse units/kg.[3] No toxicity equivalent factors have been set for brevetoxins, which for regulatory purposes are deemed to be equipotent to BTX-2.[4]
Diarrhetic Shellfish Poisoning (DSP)
DSP toxins include OA, DTX1, DTX2, DTX3 and PTX, but do not include Pectenotoxin-2 seco acids, Yessotoxins, Gymnodimne or Azaspiracids. Analysis is undertaken by Liquid chromatography–mass spectrometry/mass spectrometry (LC-MS/MS). A maximum level of greater than or equal to 0.16 mg OA eq/kg of edible shellfish flesh is applied. This is more stringent than the maximum level specified in the FSANZ Food Standards Code 1.4.1, but is consistent with recommendations by Codex Standard 292-2008 (revised 2014) and EFSA (2008)[5]. In the calculation of toxin levels, pectenotoxin is assumed to be equipotent with OA.
Toxins not regulated in Australia under the Food Standards Code
Yessotoxins (YTX)
Yessotoxins include YTX, 45OH-YTX, Homo-YTX and 45OH Homo-YTX. Analysis is undertaken by Liquid chromatography–mass spectrometry/mass spectrometry (LC-MS/MS). A maximum level of greater than or equal to 3.75 mg YTX eq/kg of edible shellfish flesh is applied. .
Azaspiracid Shellfish Poisoning (AZP)
The Azaspiracid group includes AZA-1, AZA-2 and AZA-3. Analysis is undertaken by Liquid chromatography–mass spectrometry/mass spectrometry (LC-MS/MS). A maximum level of greater than or equal to 0.16 mg AZP/kg of edible shellfish flesh is applied. This is consistent with the recommendations in Codex Standard 292-2008 (revised 2014).
Appendix 11 – Nuisance/Toxic Phytoplankton Management Protocols
Alexandrium spp.
Dinophysis acuminata, Dinophysis spp., Prorocentrum lima
Pseudo-nitzschia spp.
Rhizosolenia chunii
Gymnodinium catenatum
Karenia / Karlodinium group
Alexandrium spp.
BACKGROUND
Alexandrium spp. are small, armoured dinoflagellates. The latter are golden-brown algae with a large nucleus. They have two flagella, one protruding from a horizontal girdle groove and the other from a vertical sulcus groove (Hallegraeff, 2002).
A number of species of Alexandriumhave been found to produce a range of toxins grouped as Paralytic Shellfish Poisons (PSPs) that may be accumulated in the flesh of shellfish. PSPs may be fatal to human consumers of contaminated shellfish through respiratory paralysis although this is rare, and there have been no fatal cases in Australia. It should be noted that toxicity within a species may be variable both with locality and time. It is stressed that some Alexandrium species are difficult to identify definitively and expert assistance should be sought where doubt exists. Until definitive identification is obtained, it should be assumed that all the forms of Alexandrium present are toxic.
The symptoms of paralytic shellfish poisoning include numbness, dizziness, nausea, tingling in the extremities, vomiting and diarrhoea in mild cases (within 30 minutes), to choking sensations, breathing difficulties and death from respiratory paralysis 2 – 24 hours after ingestion in severe cases (Hallegraeff, 1997).
Very high levels of Alexandrium catenella have resulted in highly toxic shellfish (including wild mussels) in Port Phillip Bay in the past. This coupled with the nature of the toxin, results in this group of algae presenting a substantially greater potential threat to human health than all other potentially toxic species in these waters. However, it should be noted that most of the previous blooms of Alexandrium did not occur in the vicinity of any of the shellfish growing areas. The most susceptible area has been Hobson's Bay near the mouth of the Yarra River, and the main public health threat was from the recreational harvesting of mussels. Past monitoring included both the Victorian Shellfish Quality Assurance Program (VSQAP) and additional bay wide monitoring funded by the Health Department. The latter no longer occurs and due to the separation between the mussel harvesting areas and the more susceptible recreational areas nearer to the Yarra River, it is unlikely that this Victorian Marine Biotoxin Management Plan and VSOM monitoring will provide any warning of the presence of Alexandrium in the latter. PSP was detected in mussels from the Clifton Springs and Grassy Point harvesting areas in 1993 and 1994. A. tamarense was considered the most likely source of PSP in the winter of 1993 (Arnott et al, 1999).
The Alexandriumspp. known from Port Phillip Bay and Western Port in Victoria include the following. Those of major concern are the three PSP producing species. Several of these are new records for these areas being detected for the first time by phytoplankton monitoring.
Additional information concerning toxigenic species of Alexandrium in Australia may be found in Hallegraeff et al (1991) and Hallegraeff (2002). Similar information for New Zealand may be found in Chang (2004) and Rhodes (2005).
Alexandrium catenella | PSP (C1 – C4, gonyautoxins); present in Port Phillip Bay esp. Hobsons Bay |
Alexandrium tamarense | Some strains PSP (C1 – C4, gonyautoxins); can be toxic but NT in all Australian isolates so far; present in Port Phillip Bay. |
Alexandrium fundyense | PSP (C1 – C4, gonyautoxins); Port Phillip Bay material has been shown to be A. catenella |
Alexandrium minutum | PSP (mainly gonyautoxins); high PSP levels in SA; has bloomed in Port Phillip Bay in winter. |
Alexandrium ostenfeldii | Sometimes toxic in NZ, probably non-toxic in Port Phillip Bay & Aust; non blooming species |
Alexandrium pseudogonyaulax Alexandrium concavum Alexandrium insuetum Alexandrium peruvianum Alexandrium affine Alexandrium margalefi | Non-toxic Non-toxic, Port Phillip Bay, rare Non-toxic, Port Phillip Bay Non-toxic, Port Phillip Bay Non-toxic, may occur in Port Phillip Bay Non-toxic, may occur in Port Phillip Bay |
MANAGEMENT PROTOCOL
The following management protocol has been designed to facilitate the safe harvest of mussels from monitored Harvesting Areas in Port Phillip Bay (PPB) and Western Port (WP) for human consumption, and that harvesting does not occur when the mussels are affected by toxins. The protocol is based on the following key factors:
- A number of species of Alexandrium occur naturally in Port Phillip Bay and Western Port.
- Definitive identification of the various species of Alexandrium may be difficult.
- A. catenella has bloomed several times in the past in Hobson's Bay (northern Port Phillip Bay) although not in the vicinity of the shellfish harvesting areas.
- A. tamarense may have been responsible for the presence of PSP in mussel tissue in PPB in the past.
- Extreme PSP intoxication is potentially lethal to human beings.
- The potentially toxic species A. catenella, A. minutum and A. tamarense have been detected in PPB.
- Due to the status of PPB as a harbour and the presence of a substantial number of foreign species probably introduced through ballast water, there is a danger that other toxic forms will be introduced.
- Routine phytoplankton sampling for PSP producing phytoplankton will continue to occur at all Victorian Harvesting Areas.
- Analysis of mussel tissue for PSP will be undertaken if phytoplankton numbers exceed the specified phytoplankton trigger levels for biotoxin testing.
The following management protocol has been modified from the methodology used successfully by the VSQAP between August 1999 and December 2008, where routine PSP biotoxin analyses were preformed at each Harvesting Area in Port Phillip Bay and Western Port. Within the VSOM, PSP biotoxin testing will be performed when phytoplankton abundance triggers indicate this is necessary, as for other biotoxins. The VMBMP has been modified to incorporate this change in monitoring methods for the Port Phillip Bay and Western Port shellfish Harvesting Areas. It should be noted that the principal trigger for harvest suspension is the biotoxin level in shellfish tissue; phytoplankton abundance forms an additional, early warning trigger allowing precautionary closure pending biotoxin results.
- Phytoplankton samples are taken routinely at all Victorian Harvesting Areas.
- If potential toxin producing species (or unknown species) of Alexandrium are detected in a routine sample at an abundance of >200 cells/L (2 cells/mL), the following actions must be undertaken:
- A warning must be issued to all relevant harvesters.
- The sampling frequency must be reviewed with a view to increasing it to provide the relevant data.
- A tissue sample be collected and sent for PSP analysis immediately.
- If sampling frequency is not increased, then harvesting should be suspended pending the results of the next routine sampling event; previous history shows that the numbers of the phytoplankton can increase very rapidly. A live phytoplankton sample (preferably concentrated) is to be sent by overnight courier to a suitably qualified expert on this group e.g. Prof Gustaff Hallegraeff at the University of Tasmania for definitive identification. Advise the recipient in advance that the sample has been despatched.
- Where doubt exists as to the identity of the form of Alexandrium present, toxicity should be assumed until biotoxin levels are known.
- Where Alexandrium species are detected in numbers >500cells/L, harvesting should be suspended pending the results of tissue testing. The relevant analytical laboratory should be advised of the phytoplankton result and the urgency of the situation.
- Where tissue is found to contain PSPs at a level exceeding 0.8 mg/kg (80 µg/100g) tissue (the regulatory limit), harvesting is to be suspended and is to remain suspended until two successive samples taken at least 48 hours apart reveal toxin levels < 0.8 mg/kg tissue.
- Where lower levels of toxin are detected during the growth phase of a bloom, harvesting should be suspended and sampling frequency increased to monitor the development of the bloom.
- Where toxin levels have exceeded the 0.8 mg/kg tissue regulatory limit during a bloom, but the bloom is clearly degenerating, harvesting may be resumed once toxin levels remain less than 0.8 mg/kg for two successive samples taken at least 48 h apart.
- If any toxin producing Alexandrium species are present, and/or low PSP levels are detected, the frequency of sampling should be reviewed and amended to ensure that it is adequate to detect changes in either phytoplankton or biotoxin levels in an effective and timely manner.
- When harvesting is suspended during a toxic bloom, the sampling frequency may be reduced to that required by the monitoring program to save resources and costs. However, two "clear" biotoxin results ("clear" = PSP levels < 0.8 mg/kg tissue) taken at least 48 h apart are required before harvesting can resume.
- Once Alexandrium and PSP toxins are undetectable, the normal sampling regime may be resumed.
VMBMP Phytoplankton Abundance Threshold Levels (cells/L)
Phytoplankton Species | Toxin | Warning Issued to Growers | Tissue Testing | Harvest Suspension (Pending Toxin Analysis) | Harvest Resumption |
Alexandrium catenella | PSP | 200 | 200 | 500 | <0.8 mg/kg PSP for 2 successive samples over 48 hours and phytoplankton abundance not rising. |
Alexandrium minutum | PSP | 200 | 200 | 500 | As Above |
Alexandrium tamarense | PSP Some strains | 200 | 200 | 500 | As Above |
Alexandrium spp. (unknown or in doubt) | PSP Some strains | 200 | 200 | 500 | As Above |
PSP regulatory limit: 0.8 mg saxitoxin equivalents/kg tissue | |||||
REFERENCES
Arnott, G.H., Reilly, D.J. and Werner, G.F. (1999). Victorian Shellfish Quality Assurance Program. 7. Sanitary Survey Update: Clifton Springs and Grassy Point (Portarlington) Aquaculture Zones. Marine and Freshwater Resources Institute, Report No. 13, 1 – 36
ASQAAC (2006). Australian Shellfish Quality Assurance Program Operations Manual. Developed by the Australian Shellfish Quality Assurance Advisory Committee.
Chang, F.H. (2004). A Review of the Currently Monitored Toxigenic Phytoplankton Species in New Zealand. Report Prepared for New Zealand Food Safety Authority by National Institute of Water & Atmosphere Pty Ltd (NIWA). Report WLG2004-80.
Hallegraeff, G.M., Bolch, C.J., Blackburn, S.I. & Oshima, Y (1991). Species of the Toxigenic Dinoflagellate Genus Alexandrium in Southeastern Australian Waters. Botanica Marina, 34: 575 – 587.
Hallegraeff, G. (1997). Algal toxins in Australian Shellfish. In: Foodborne Microorganisms of Public Health Significance. Fifth Edition. AIFST (NSW Branch), Food Microbiology Group.
Hallegraeff, G. (2002). Aquaculturists' Guide to Harmful Australian Microalgae. The Print Centre, Hobart.
Rhodes, L. (2005). Response to a Review of the Currently Monitored Toxigenic Phytoplankton Species in New Zealand. Cawthron report No. 979: 1-18
Todd, K. (2001). Australian Victorian Marine Biotoxin Management Plan for Shellfish Farming. Prepared for the Australian Shellfish Quality Assurance Committee (ASQAAC) by the Cawthron Institute. Cawthron Report No. 645
Dinophysis acuminata and Dinophysis spp.
BACKGROUND
Dinophysis are large, bag shaped dinoflagellates with well-developed sulcal lists and reduced epitheca (Hallegraeff, 2002). They are common in Australian waters but not often abundant.
Some Dinophysisspecies have been found to produce fat-soluble polyether compounds called Diarrhetic Shellfish Poisons (DSPs) that may accumulate in the shellfish that consume them. DSPs may cause illness in human consumers of contaminated shellfish.
The major symptoms of DSP poisoning are diarrhoea, vomiting, nausea and abdominal pain. There is also some evidence of tumour formation in the digestive system as a result of chronic exposure. Recovery occurs after about 3 days irrespective of medical treatment (Hallegraeff, 1997) and no human fatalities have been recorded.
Following the characterisation of okadaic acid (OA), okadaic acid derivatives were isolated from shellfish including the dinophysis toxins (DTX) incorporating the OA esters (DTX 3). Subsequently, other toxins have been included in this group despite their different chemical structures and modes of action. These include the pectenotoxins (PTX), pectenotoxin seco acids (PTX2-SA) and yessotoxins (YTX). Recent work in Australia by Burgess (2002), and in New Zealand for the Marlborough Sounds Shellfish Quality Program by MacKenzie (2002), has shown that PTX2-SA compounds are not toxic to humans. Consequently, for the purposes of the Victorian Marine Biotoxin Management Plan (VMBMP), PTX2–SAs are no longer regulated as a DSP toxin. Diarrhoegenic effects have been demonstrated only for OA and DTX; PTX 1-4 have been found to cause liver damage and YTX damages cardiac muscle in mice (Hallegraeff, 1997).
The main bloom species in Port Phillip Bay (PPB) and Western Port (WP) appears to be Dinophysis acuminata. Dinophysis acuminatain Australian and New Zealand waters has not been found to produce significant amounts of OA or DTX, although it does produce significant amounts of PTX 2 that seems to be rapidly converted to non-toxic PTX2-SA in mussels. Mussels from PPB and WP have been tested for DSP revealing that PTX2-SA predominated with very small amounts of PTX2 and traces of OA. No DTX 1 or 2 was found. DTX 3 (OA esters) has not been tested to date but will be in future. Despite the consumption of large quantities of mussels from the aquaculture reserves in PPB and WP, there has never been a report of a case of diarrhetic shellfish poisoning.
The Commission of European Communities (CEC) has published draft regulations covering DSP toxins in shellfish where it is proposed that a limit of 16 µg/100g total DSP content including OA, DTXs and PTXs (CEC, 2001; Holland et al, 2002) be adopted. This is despite the fact that the EU expert working group on all fat-soluble marine algal toxins (2001) removed both the PTXs and YTXs from this group (Aune in MacKenzie, 2002). The later group suggested a limit for PTX of 15 g/100g. PTX is still regarded as toxic and although currently included as a DSP for the VVMBMP, should be regulated separately. The FSANZ Food Standards Code regulatory limit for DSP is adopted for the VMBMP.
YTX and azaspiracids (AZA) which are included by some as DSPs but are chemically distinct, are not regulated in Australia. For the purposes of the VMBMP, these compounds are not considered to be members of the DSP group of toxins. The oral toxicity of the YTXs is questionable and neither YTXs or AZAs has been detected in Australian shellfish to date. Currently, these compounds are analysed using the LC-MS/MS method for DSP toxins. Within the VMBMP, when DSP biotoxin testing is performed, qualitative testing is also carried out for YTX and AZA. Once standards are available, and quantitative analysis is possible, regulation within the VMBMP will be considered. At this time, the EU draft regulations have recommend limits for both YTX and AZA.
The species of Dinophysis known to be or likely to be recorded in PPB and WP are:
Dinophysis acuminata (produces principally PTX-2-SA in PPB)
Dinophysis fortii (potential DSP producer in PPB)
Dinophysis caudata (potential DSP producer but not recorded in PPB at this time)
Dinophysis acuta (potential DSP producer but rare in Australia)
Dinophysis tripos (potential DSP producer, widespread in Australia but rare)
Dinophysis hastata (potential DSP producer, widespread in Australia but rare)
Since August 1999, only Dinophysis acuminata has occurred in numbers sufficient to initiate biotoxin sampling/analysis within harvesting areas in Port Phillip Bay (PPB) and Western Port.
Further information concerning the toxicity of Dinophysis spp in New Zealand may be found in Chang (2004) and Rhodes (2005).
MANAGEMENT PROTOCOL
This management protocol specifically relates to Dinophysis acuminata where abundance and biotoxin data has been collected over several years but all other species are treated in the same way until additional information indicates otherwise. Most species do not generally occur in numbers sufficient to cause concern in Port Phillip Bay and Western Port. It is designed to ensure that mussels harvested from these waters are safe for human consumption and that harvesting does not occur when the mussels are affected by DSP toxins produced by various Dinophysis species. It is based on the following key factors:
- Various species of Dinophysis have been detected in Port Phillip Bay and Western Port, notably D. acuminata.
- A number of species are known to produce significant DSP levels in shellfish tissue at very low abundances.
- Dinophysis acuminata in Port Phillip Bay produces PTX2-SA but little OA & PTX and no DTX. OA esters (DTX 3) have not been tested for to date.
- PTX2-SA has been shown to be non-toxic to humans and is excluded as a DSP toxin.
- Due to its low abundance trigger, the numbers of D. acuminata can vary quickly between levels above and below the trigger level, making management difficult.
- The relationship between tissue DSP levels and D. acuminata numbers is poor.
- The symptoms of DSP are relatively minor and human deaths have never occurred.
- The trigger for D. acuminata is likely to be conservative and it has been found to be less toxic than some other Dinophysis such as D. acuta in New Zealand (Rhodes, 2005).
- Routine phytoplankton sampling for DSP producing phytoplankton will continue to occur at all Victorian Harvesting Areas.
- Analysis of mussel tissue for DSP will be undertaken if phytoplankton numbers exceed the specified phytoplankton trigger levels for biotoxin testing.
Tissue testing is based on phytoplankton triggers as DSP is not routinely tested for in the VMBMP. The following management protocol has been used successfully within the former VSQAP from August 1999 to December 2008 for the management of Dinophysis acuminata blooms. It has been adopted for the current Victorian Marine Biotoxin Management Plan (VMBMP) including the mussel growing areas in Port Phillip Bay and Western Port. It should be noted that the principal trigger for harvest suspension is the biotoxin level in shellfish tissue; phytoplankton abundance forms an additional, early warning trigger allowing precautionary closure pending biotoxin results.
- Phytoplankton monitoring is carried out routinely at each Harvesting Area.
- It is recommended that more rapid sedimentation methods than gravity be used to concentrate samples for counting Dinophysis due to the necessity for a rapid turnaround time. It is also recommended that the concentration factor be X10 more than that usually used for counting algae due to the very low threshold value for Dinophysis and the necessity for greater accuracy at low abundance levels.
- If Dinophysis acuminata numbers exceed 1,000 cells/L, a tissue sample should be collected immediately, shucked and sent to the Advanced Analytical Australia for DSP biotoxin analysis. Qualitative analysis for YTX and AZA can also be performed at the Cawthron Institute. or the Queensland Health Laboratories.
- If Dinophysis acuminata numbers exceed 2,000 cells/L, growers are to be notified and a voluntary suspension of harvesting implemented pending the biotoxin analysis.
- If the total DSP level in mussel tissue (OA, DTX and PTX but excluding PTX2-SA) exceeds the regulatory limit of 0.16 mg/kg (16 µg/100g) tissue, harvesting should be suspended and sampling frequency increased. The latter is very important as the abundance of Dinophysis acuminata can vary from problem to non-problem levels within days.
- If the phytoplankton monitoring indicates that a D. acuminata bloom is developing (trend of increasing numbers), then the monitoring frequency should be increased and harvesting suspended if DSP levels exceed the Food Standards Code Regulatory Limit.
- Once harvesting has been suspended due to the presence of DSP in mussel tissue, harvesting may not be resumed until two successive "clear" biotoxin results are obtained at least 48 hours (2 days) apart. In this case, a clear biotoxin result means DSP levels less than 0.16 mg/kg (16 µg/100g) tissue.
- Because the abundance of Dinophysis spp. can rise and fall rapidly above and below the threshold levels for tissue testing and the suspension of harvesting, this may be a difficult situation to manage once closure has been initiated. This will also be complicated by the fact that abundance may not only vary rapidly with time, but also between sites.
- Other Dinophysis species should be dealt with using the same thresholds as for D. acuminata, until more information is gathered on their toxin production and toxicity.
- The abundance threshold values and biotoxin regulatory limits for Dinophysis spp. should be updated regularly as new information becomes available. This is particularly the case with DTX 3 and Dinophysis acuminata.
VMBMP Phytoplankton Abundance Threshold Levels (cells/L)
Phytoplankton Species | Toxin | Warning Issued to Growers | Tissue Testing | Harvest Suspension (Pending Toxin Analysis) | Harvest Resumption |
Dinophysis acuminata | DSP | 1,000 | 1,000 | 2,000 | <0.16 mg/kg DSP for 2 successive samples taken not < 48 hours (2 days) apart; phytoplankton abundance not rising. |
Dinophysis caudata | DSP | 1,000 | 1,000 | 2,000 | As Above |
Dinophysis acuta | DSP | 500 | 500 | 1,000 | As above |
Dinophysis fortii | ?DSP | 1,000 | 1,000 | 2,000 | As Above |
Dinophysis spp. | ?DSP | 500 | 500 | 1,000 | As Above – precautionary only till further information available |
Prorocentrum lima | ?DSP | 1,000 | 1,000 | 2,000 | <0.16 mg/kg DSP for 2 successive samples taken not < 48 hours (2 days) apart; phytoplankton abundance not rising. |
DSP REGULATORY LIMIT: 0.16 mg OA equivalents/kg tissue | |||||
Prorocentrum lima
Prorocentrum limais another dinoflagellate which is oval in shape and bears a small anterior indentation. It has been recorded widely over southern Australia including in Port Phillip Bay, the Gippsland Lakes and Tasmania. It is a benthic or epibenthic species commonly found attached to seaweeds and shallowly in sand (Hallegraeff, 2002).
This species has been found to produce DSP overseas (specifically OA and DTX-1), including in New Zealand. However, its toxicity status in Australia is uncertain and a culture from WA was found to be non-toxic.
For the purposes of the VMBMP, it has been assumed that this species is a DSP producer similar to Dinophysis acuminata, and the abundance and biotoxin triggers utilised for Dinophysis acuminata have been adopted until more information becomes available. Hence the management protocol for Dinophysis should be utilised for this species as well.
REFERENCES
ANZFA (2002). Food Standards Code. Australian and New Zealand Food Authority
Chang, F.H. (2004). A Review of the Currently Monitored Toxigenic Phytoplankton Species in New Zealand. Report Prepared for New Zealand Food Safety Authority by National Institute of Water & Atmosphere Pty Ltd (NIWA). Report WLG2004-80.
Commission of European Communities (2001). Draft Commission Decision of Establishing the Methods of Analysis and the Maximum Limits fro Certain Marine Biotoxins in Bivalve Molluscs, Echinoderms, Tunicates and Marine Gastropods. Brussels, SANCO/2227/2001 Rev 3
Hallegraeff, G. (1997). Algal toxins in Australian Shellfish. In: Foodborne Micro-organisms of Public Health Significance. Fifth Edition. AIFST (NSW Branch), Food Microbiology Group.
Hallegraeff, G. (2002). Aquaculturists' Guide to Harmful Australian Microalgae. The Print Centre, Hobart.
Holland, P.T., McNabb, P., Selwood, A., Page, T., Bell, K and MacKenzie, L. (In Press). Marine Biotoxin Monitoring of New Zealand Shellfish – A New Management Programme Based on LC-MS. In: Proc. 2PndP Int. Conference on Harmful Algae Management and Mitigation. Nov 2001, Qingdao, China. Hall, S. & Zou, YL (Ed.)
MacKenzie, L. (2002). An Evaluation of the Risk to Consumers of Pectenotoxin 2 seco acid (PTX2-SA) Contamination of GreenshellÔ Mussels. Prepared for Marlborough Sounds Shellfish Quality Programme. Cawthron Institute Report 750, 1- 50
MacKenzie, L., Holland, P., McNabb, P., Beuzenberg, V., Selwood, A. and Suzuki, T. (2002). Complex Toxin Profiles in Phytoplankton and Greenshell Mussels (Perna canaliculus), Revealed by LC-MS/MS Analysis. Toxicon, 40: 1321 – 1330
Rhodes, L. (2005). Response to a Review of the Currently Monitored Toxigenic Phytoplankton Species in New Zealand. Cawthron report No. 979: 1-18
Todd, K. (2001). Australian Victorian Marine Biotoxin Management Plan for Shellfish Farming. Prepared for the Australian Shellfish Quality Assurance Committee (ASQAAC) by the Cawthron Institute. Cawthron Report No. 645
Karenia/Karlodinium group
Karenia
brevis
Karenia spp
Karlodinium
micrum
Gymnodinium
impudicum
BACKGROUND
Karenia spp. are small, unarmoured dinoflagellate phytoplankton (golden-brown algae with a large nucleus). They have two flagella, one protruding from a horizontal girdle groove and the other from a vertical sulcus groove (Hallegraeff, 2002).
Many species belonging to this and related genera are common in Australian and New Zealand waters. When in bloom, a significant number of species within this group are fish killers, particularly where fish cannot avoid the blooms. Despite this, the vast majority don't appear to cause any adverse reactions in humans who consume shellfish from bloom areas in Australia or New Zealand.
It is stressed that the taxonomy of this group is poorly understood although this situation has improved recently with an increase in the research being carried out in New Zealand and Australia. Species of Karenia may be very difficult to identify definitively using light microscopy and expert assistance should be sought. Identification is best done with live material.
Karenia brevis in Florida which is principally associated with fish kills, produces brevetoxins (BTX) that may cause non-fatal but unpleasant neurological symptoms in humans exposed to them by direct contact (e.g.swimming through blooms), inhalation (e.g.near fish kills or breaking waves containing blooms) or through the consumption of contaminated shellfish. For mild cases of intoxication, the symptoms include chills, headache, diarrhoea, muscle weakness, muscle and joint pain and vomiting 3 – 6 hours after exposure. In extreme cases, other symptoms may occur including paraesthesia, altered perception of hot and cold, difficulty breathing, double vision, and trouble talking and swallowing (Hallegraeff 1997, 2002). There have been no fatalities associated with NSP intoxication.
It is doubtful that Karenia brevis sensu strictu is present in Australia or New Zealand but a group of morphologically similar species are. Approximately 180 cases of shellfish poisoning occurred in New Zealand in 1993. It was concluded that the symptoms experienced by these people after consuming shellfish, were most likely caused by an NSP toxin. However, it was also noted that other toxins were present apart from BTX including DSP, and that microbiological contamination may have played a role in the illness (Todd, 2000). It was later found that what was reported as Karenia cf brevis or Gymnodinium cf mikimotoi at that time may have contained four or more gymnodinioid species including what, in New Zealand, have been called Karenia brevisulcataand K. sellifromis as well as Karenia mikimotoi, Karenia bidigitata and Gymnodinium aureolum. At the 18th Marine Biotoxin Science Workshop (2001) in the New Zealand, the consensus was that K. mikimotoi was the dominant organism present, although it is still unclear exactly what other species were present during the event and which was/were responsible for the toxication.
Karenia mikimotoiis very common in Victorian waters and has been associated with fish kills. Like other species resembling K. brevis, it is a flattened species although much less so in extent. It has never been associated with human toxicity in Australia despite huge blooms of it including in the waters of shellfish harvesting areas, and large quantities of mussels have been consumed from areas where it was present.
There has only been a single shellfish poisoning incident attributed to NSP in Australia occurring in Gippsland, Victoria in 1994. This resulted from the consumption of wildstock mussels from the Tamboon Inlet. Karenia cf brevis was identified as the causative organism (Arnott, 1998; Todd, 2001). Karenia cf brevis has also been recorded in Port Phillip Bay (PPB) twice at the Clifton Springs Harvesting Area as part of the former Victorian Shellfish Quality Assurance Program (VSQAP) phytoplankton monitoring, in numbers up to 32,000 cells/L (Arnott et al 1999), but there have been no reports of any type of shellfish poisoning over that period. Whether this was the same species as that at the Tamboon Inlet or another species resembling K. brevis is not known.
In the USA, K. mikimotoi produces only about one third as much BTX as K. brevis (Todd, 2002) and New Zealand isolates produce much lower levels than these. In Florida, shellfish harvesting is suspended when K. brevis numbers reach 5,000 cells/L (Hallegraeff, 2002).
Other species such as Karenia selliformis from New Zealand are known to be associated with fish kills and produce ichthyotoxins including gymnodimine. Gymnodimine is not a risk to human health and does not produce neurotoxic shellfish poisoning, although it can kill mice during bioassays. Although there is little evidence that these species are toxic to human consumers of shellfish, the ichthyotoxins they produce are not well understood and further local information should be gathered.
BTX testing by mouse bioassay is not routinely carried out in Australia currently, but is in New Zealand. However, its interpretation is complex and can be complicated by the effects of other marine toxins and related compounds such as gymnodimine, Wellington Harbour Toxin and fatty acids naturally found in shellfish that may kill mice during bioassay but NOT indicate human toxicity from NSP (false +ve). The Cawthron Institute in New Zealand has recently developed more definitive methods LC/MS methods for NSP analysis that will improve the management of this biotoxin. and eventually replace the current ether mouse bioassay (Todd, 2002).
Work by the Cawthron Institute in New Zealand using this LC-MS analysis has failed to confirm BTX production in any of the Karenia species tested in that country, including K. mikimotoi.
At this time, within Port Phillip Bay and Western Port (WP) few, if any, Karenia species, including flattened species similar to K. brevis and K. mikimotoi, seem to offer any marked potential for human toxicity from the consumption of shellfish. The risk appears slight but until more information is known, their presence should be monitored and toxin testing performed when the threshold abundance levels are exceeded.
The potentially toxic Kareniaknown from southern Australia, and in particular from Port Phillip Bay and Western Port in Victoria include the following species, although New Zealand work shows that if a number of these species is toxic, toxicity is very low. Due to the uncertain state of the taxonomy of this group, some other fish kill species not yet found in Port Phillip Bay, Western Port or Australia have been listed. Out of this group of dinoflagellates, the Australian Victorian Marine Biotoxin Management Plan for Shellfish Farming (2001) lists only Karenia cf brevis in its phytoplankton abundance trigger table. The more recent New Zealand Phytoplankton Action Levels (May 2005) have been adopted use in for the VMBMP. The FSANZ Food Standards Code regulatory limit for NSP is adopted for the VMBMP.
Karenia brevis (NSP – BTX; Unlikely to be present in Aust.)
Karenia cf brevis (?NSP; Flattened species like K. brevis; PPB, Gippsland, NZ)
Karenia mikimotoi (low levels NSP; Fish kills; widespread incl. PPB, Gippsland Lakes.
Karenia papilionacea (low levels NSP - New Zealand; fish kills)
Karenia selliformis (NSP (gymnodimine) - New Zealand; fish kills)
Karenia bidigitatum (low levels NSP - New Zealand; fish kills)
Karenia digitata (fish kills; Hong Kong Harbour)
Karenia cf longicanalis (fish kills, toxin?; Hong Kong, Tasmania (similar sp.)
Karenia brevisulcata (fish kills, "Wellington Harbour" Toxin; Wellington Harbour only, NZ)
(uncharacterised toxin? No BTX)
Karlodinium micrum (fish kills, Australia, New Zealand)
Gymnodiinium impudicum ?? on NZ list
MANAGEMENT PROTOCOL
The following management protocol relates to Karenia brevis, the Karenia spp listed (including flattened Australian species similar to K. brevis), Karlodinium micrum and Gymnodinium impudicum. It has been designed to ensure that mussels harvested from Port Phillip Bay and Western Port are safe for human consumption, and that harvesting does not occur when the mussels are affected by biotoxins. The protocols are based on the following key factors:
- A number of species of Karenia and related genera occur naturally in Port Phillip Bay and Western Port.
- Definitive identification of the various species of Karenia may be difficult.
- A form of Karenia in PPB has been identified as Karenia cf brevis (=Gymnodinium cf breve) in the past although the state of knowledge of the taxonomy of this group was incomplete at that time.
- Large blooms of Karenia mikimotoi have been recorded from Port Phillip Bay which were responsible for massive fish kills in 1950's
- There is no evidence of an incident of NSP intoxication in Port Phillip Bay or Western Port despite blooms of Gymnodinium/Karenia occurring in the past, including Karenia mikimotoi and Karenia cf brevis.
- Based on New Zealand experiences, there may be a risk of NSP from Karenia brevis as Port Phillip Bay contains major ports and there is a real risk this species may become introduced to Australia. There is also a slight risk from Karenia cf brevis (other, flat Karenia species that resemble K. brevis) and the species listed above.
- In Florida, shellfish harvesting is banned when K. brevis abundance reaches 5,000 cells/L (Hallegraeff, 2002). K. mikimotoi produces only a third as much toxin in the USA as K. brevis, and even less in New Zealand. The K. brevis threshold levels recommended in the Australian Victorian Marine Biotoxin Management Plan for Shellfish Farming of 1,000 cells/L for tissue testing and 5,000 cells/L for voluntary harvesting suspension and the issue of public health warnings seem very conservative in the light of recent advances in New Zealand. Consequently, the New Zealand Phytoplankton Action Levels for Karenia spp and related forms have been adopted for the VMBMP.
- There is no evidence that the other fish killing species can cause human intoxication from to the consumption of shellfish.
- Routine phytoplankton sampling for NSP producing phytoplankton will continue to occur at all Victorian Harvesting Areas.
- Analysis of mussel tissue for NSP will be undertaken if phytoplankton numbers exceed the specified phytoplankton trigger levels for biotoxin testing.
The following management protocol adopts the New Zealand NZFSA alert levels (May 2005). Expert assistance may be required to identify the relevant species. It has been used successfully within the VSQAP between August 1999 and December 2008 and has been adopted for the current Victorian Marine Biotoxin Management Plan (VMBMP) including the mussel harvesting areas in Port Phillip Bay and Port Phillip Bay. It should be noted that the principal trigger for harvest suspension is the biotoxin level in shellfish tissue; phytoplankton abundance forms an additional, early warning trigger allowing precautionary closure pending biotoxin results and as a trigger for biotoxin testing.
- If what is or suspected to be Karenia brevis is detected in a routine sample at an abundance of >1,000 cells/L (1 cell/mL), a warning should be issued to the relevant growers. A live phytoplankton sample (preferably concentrated) is to be sent by overnight courier to Prof Gustaff Hallegraeff at the University of Tasmania (or other suitably qualified experts in the group) for definitive identification. Advise Prof Hallegraeff in advance the sample has been despatched.
- If the species is confirmed as K. brevis, a mussel tissue sample must also be collected and sent to the Cawthron Institute for biotoxin analysis. In most cases, mussel tissue samples will already have been collected during routine sampling in case phytoplankton monitoring indicated biotoxin analysis was required. In this case, the tissue should be prepared and then dispatched to the analytical laboratory as quickly as possible.
- The sampling frequency should be reviewed to ensure it is adequate to detect a rapid increase in phytoplankton numbers.
- If there is uncertainty concerning the identification as Karenia brevis NSP toxicity should be assumed until biotoxin levels are known.
- Where Karenia brevis is detected in numbers >2,000cells/L, harvesting should be suspended pending the results of tissue testing. The laboratory should be advised of the phytoplankton result and the urgency of the situation.
- Where any of the other Karenia species noted above (NOT K. brevis) are detected (there may be more than 1 species present) in numbers exceeding 100,000 cells/L, growers should be notified and definitive identifications obtained.
- If the numbers exceed 250,000 cells/L, a mussel tissue sample must also be collected and sent to the Cawthron Institute for biotoxin analysis (see Step 2 above).
- If numbers rise above 300,000, harvesting should be suspended pending the results of biotoxin testing.
- Where tissue is found to contain NSP (BTX) at a level exceeding 200 MU/kg (20 MU/100g) tissue (the regulatory limit) or 0.8BTX-2 eq mg/kg, harvesting is to be suspended and is to remain suspended until two successive samples taken at least 48 hours (2 days) apart reveal toxin levels < 200 MU/kg or < 0.8BTX-2 eq mg/kg tissue.
- Where lower levels of toxin are detected during the growth phase of a bloom, harvesting should be suspended and sampling frequency increased to monitor the development of the bloom.
- Where toxin levels have exceeded the 200 MU/kg or 0.8BTX-2 eq mg/kg tissue regulatory limit during a bloom, but the bloom is clearly degenerating, harvesting may be resumed once toxin levels remain less than 200 MU/kg or 0.8BTX-2 eq mg/kg for two successive samples taken at least 48 hours (2 days) apart.
- If Karenia brevis or Karenia spp are present in numbers approaching their trigger levels and/or low NSP levels are detected, the phytoplankton and biotoxin sampling frequency should be revised to ensure adequate monitoring of any bloom that may develop.
- When harvesting is suspended during a toxic bloom, the sampling frequency may be reduced to the routine fortnightly monitoring program to save resources and costs. However, two "clear" biotoxin results ("clear" = < 200 MU/kg or 0.8BTX-2 eq mg/kg tissue) at least 48 hours (2 days) apart are required before harvesting can resume.
- Once Karenia brevis, or Karenia spp abundance is clearly less than the trigger values and NSP toxins are undetectable, the routine fortnightly sampling regime may be resumed.
VMBMP Phytoplankton Abundance Threshold Levels (cells/L)
Phytoplankton Species | Toxin | Warning Issued to Growers | Tissue Testing | Harvest Suspension (Pending Toxin Analysis) | Harvest Resumption |
Karenia brevis (Not currently recorded in Australia) | NSP (BTX) | 1,000 | 2,000 | 5,000 | <200 MU/kg or 0.8BTX-2 eq mg/kg for two successive samples taken at least 2 days apart; phytoplankton abundance not rising |
Karenia mikimotoi, K. papilionacea, K. bidigitata, K. brevisulcata, K. selliformis Flattened Australian species morphologically similar to K. brevis or K. mikimotoi Karlodinium micrum, Gymnodinium impudicum | |||||
| ?NSP | 100,000 | 250,000 | 300,000 | As Above |
NSP REGULATORY LIMIT: 200 MU/kg or 0.8BTX-2 eq mg/kg tissue | |||||
REFERENCES
ANZFA (2002). Food Standards Code. Australian and New Zealand Food Authority
Arnott, G.H. (1998). Toxic Marine Microalgae: A worldwide Problem With Major Implications for Seafood Safety. Advancing Food Safety 1: 24 - 34
Arnott, G.H., Reilly, D.J. and Werner, G.F. (1999). Victorian Shellfish Quality Assurance Program. 7. Sanitary Survey Update: Clifton Springs and Grassy Point (port Arlington) Aquaculture Zones. Marine and Freshwater Resources Institute, Report No. 13, 1 – 36
Chang, F.H. (2004). A Review of the Currently Monitored Toxigenic Phytoplankton Species in New Zealand. Report Prepared for New Zealand Food Safety Authority by National Institute of Water & Atmosphere Pty Ltd (NIWA). Report WLG2004-80.
Hallegraeff, G. (1997). Algal toxins in Australian Shellfish. In: Foodborne Microorganisms of Public Health Significance. Fifth Edition. AIFST (NSW Branch), Food Microbiology Group.
Hallegraeff, G. (2002). Aquaculturists' Guide to Harmful Australian Microalgae. The Print Centre, Hobart.
Rhodes, L. (2005). Response to a Review of the Currently Monitored Toxigenic Phytoplankton Species in New Zealand. Cawthron report No. 979: 1-18
Todd, K. (2001). Australian Victorian Marine Biotoxin Management Plan for Shellfish Farming. Prepared for the Australian Shellfish Quality Assurance Committee (ASQAAC) by the Cawthron Institute. Cawthron Report No. 645
Todd, K. (2002). A Review of NSP Monitoring in New Zealand In Support of a New Program. Prepared for the Marine Biotoxin Technical Committee, New Zealand. Cawthron Report 660: 1 - 30
Pseudo-nitzschia spp.
BACKGROUND
Pseudo-nitzschiaspp. are narrow, elongate diatoms that are difficult to identify to species level using light microscopy; generally, electron microscopy is required.
A number of species, notably P. multiseries and P. australis, have been found to produce the Amnesic Shellfish Poison (ASP) domoic acid that may be accumulated in the flesh of shellfish. ASPs may cause illness in people consuming contaminated shellfish such as mussels, oysters and scallops. It should be noted that toxicity within a species may be variable both with locality and time. Until definitive identification is obtained, it should be assumed that all the forms of Pseudo-nitzschiapresent are toxic.
A serious shellfish-poisoning outbreak in humans in Canada in 1987 resulted in memory loss in extreme cases of intoxication, and consequently, the syndrome was called Amnesic Shellfish Poisoning (ASP). The causative compound was found to be domoic acid. The symptoms of ASP are nausea, vomiting, diarrhoea and abdominal cramps after 3 – 5 hours. In extreme cases there may be a decreased reaction to deep pain, dizziness, hallucinations, confusion, short-term memory loss and seizures (Hallegraeff, 1997, 2002). A small number of deaths have occurred in Canada with immuno-depressed patients most at risk. There is evidence that the concentration of domoic acid in shellfish may be species dependant with scallops most at risk and mussels much less so. There are no documented cases of amnesic shellfish poisoning in Australia. Domoic acid has not been detected in Victorian mussels since the commencement of the Victorian Shellfish Quality Assurance Program (VSQAP) in 1987, but has been detected in scallops from Bass Strait (Arnott et al, 1999).
The Pseudo-nitzschiaspecies currently known from Port Phillip Bay (PPB) and Western Port (WP) in Victoria include the following. Several of these are new records for these areas being detected for the first time by previous VSQAP phytoplankton monitoring.
Pseudo-nitzschia multiseries (potentially toxic)
Pseudo-nitzschia australis (potentially toxic)
Pseudo-nitzschia pungens (non-toxic in PPB)
Pseudo-nitzschia delicatissima (non-toxic in PPB)
Pseudo-nitzschia pseudodelicatissima (non-toxic in PPB, mildly toxic in Derwent R.)
Pseudo-nitzschia heimii (non-toxic)
Pseudo-nitzschia fraudulenta (non-toxic)
Additional information concerning Australian Pseudo-nitzschia and their toxicity may be found in Hallegraeff (1994) and Lapworth et al (2000), and for New Zealand in Chang (2004) and Rhodes (2005).
ASP (domoic acid) biotoxin sampling and analysis is carried out at Victorian Harvesting Areas when phytoplankton abundance triggers are exceeded.
MANAGEMENT PROTOCOL
The following management protocol has been designed to ensure that mussels harvested from Port Phillip Bay and Western Port are safe for human consumption, and that harvesting does not occur when the mussels are affected by ASP toxins. The protocol is based on the following key factors:
- Pseudo-nitzschia spp. are present as a component of the phytoplankton communities in Port Phillip Bay and Western Port for much of the year. They rarely form the dominant algal group within these communities (i.e. rarely > 50% of the total phytoplankton).
- The major blooms of this genus generally consist of P. pseudodelicatissima, P. delicatissima and P. pungens all of which have been found to be non-toxic in Port Phillip Bay.
- Definitive identification of the various species of Pseudo-nitzschia may require electron microscopy, and they are therefore managed as a genus (group of species).
- P. heimii (non-toxic) and the potentially toxic species P. australis and P. multiseries have been detected as minor components of Pseudo-nitzschia blooms in Port Phillip Bay.
- There is a risk that the potentially toxic species P. australis and/or P. multiseries may become a major component of blooms.
- Due to the status of Port Phillip Bay and Western Port as harbours and the presence of a substantial number of foreign species in the former, probably introduced via ballast water, there is a danger that other toxic forms or species of Pseudo-nitzschia will be introduced.
- There is a risk that if environmental conditions alter in Port Phillip Bay, currently non-toxic Pseudo-nitzschia species may become toxic.
- Routine phytoplankton sampling for ASP producing phytoplankton will continue to occur at all Victorian Harvesting Areas.
- Analysis of mussel tissue for ASP will be undertaken if phytoplankton numbers exceed the specified phytoplankton trigger levels for biotoxin testing.
The following management protocol has been used successfully within the VSQAP between August 1999 and December 2008 for the management of Pseudo-nitzschia blooms. It has been adopted for the current Victorian Marine Biotoxin Management Plan (VMBMP) including the mussel harvesting areas in Port Phillip Bay and Port Phillip Bay. It should be noted that the principal trigger for harvest suspension is the biotoxin level in shellfish tissue; phytoplankton abundance forms an additional, early warning trigger allowing precautionary closure pending biotoxin results and as a trigger for biotoxin testing.
- If Pseudo-nitzschia spp. are detected in numbers less than 100,000 cells/L (100 cells/mL), no further action but monitor numbers. Report presence to growers.
- If Pseudo-nitzschia spp. are detected in numbers greater than 100,000 cells/L (100 cells/mL) for the first time in a bloom, send part of the concentrated phytoplankton sample taken (or a duplicate sample) to an expert in this group (e.g. Prof Gustaff Hallegraeff at the University of Tasmania) for definitive species identification and clarification of the relative abundance of the species present.
- If Pseudo-nitzschia spp. (all species) are detected in numbers greater than 300,000 cells/L (300 cells/mL), or the numbers of P. australis plus P. multiseries exceeds 100,000 cells/L, institute ASP biotoxin testing as part of the routine sampling program. This analysis is additional to the current monitoring program.
- If numbers (all species) exceed 500,000 cells/L, or the numbers of P. australis plus P. multiseries exceed 300,000, suspend harvesting pending the biotoxin analysis results.
- If ASP is not detected, harvesting may be resumed immediately.
- Continue ASP analysis as part of the fortnightly routine program at the affected harvesting areas until Pseudo-nitzschia spp. levels drop below 300,000 cells/L (300 cells/mL), or in the case of P. australis plus P. multiseries, below 100,000 cells/L.
- Continue to monitor phytoplankton levels, and during each month of the bloom, send a concentrated sample to the University of Tasmania for species level identification in case the species mix varies with time.
- If the main components of a bloom are found to be species known to be non-toxic, such as P. pungens, P. delicatissima and P. pseudodelicatissima, and ASP analysis is negative, continue to repeat steps 6 and 7 until the bloom degenerates.
- If any domoic acid is detected, it is recommended an industry warning be released and sampling frequency be increased to weekly.
- If domoic acid is detected at levels > 20 mg domoic acid/kg tissue (the regulatory limit), harvesting should be suspended and sampling frequency be amended with a view to increasing it to weekly.
- Harvesting areas remain closed until domoic acid levels <20 mg/kg (<20µg/g) of tissue are found on two successive sampling occasions at least 14 days (two weeks) apart.
- It should be noted that the risk from toxic Pseudo-nitzschia remains high for two weeks after the post bloom crash.
- Once Pseudo-nitzschia levels drop below the relevant triggers and ASP is undetected in shellfish, ASP sampling/analysis and additional sampling could be discontinued. The routine fortnightly phytoplankton analysis would continue as usual.
- Re-evaluate the Pseudo-nitzschia trigger levels as more ASP testing is completed and related to Pseudo-nitzschia abundance over the period of the program.
VMBMP Phytoplankton Abundance Threshold Levels (cells/L)
Phytoplankton Species | Toxin | Definitive Identification & Warning to Growers | Tissue Testing | Harvest Suspension (Pending Toxin Analysis) | Harvest Resumption |
Pseudo-nitzschia spp. (pseudodelicatissima group & pungens) | ASP (domoic acid) | 100,000 | 500,000 | 500,000 | <20 mg/kg domoic acid for 2 successive samples over 14 days; phytoplankton abundance not rising. |
Pseudo-nitzschia australis & multiseries (in seriata group)
| ASP | 100,000 | 100,000 | 300,000 | As Above |
ASP REGULATORY LIMIT: 20 mg domoic acid /kg tissue | |||||
GENERAL
It is stressed that this protocol is specifically designed for use within Port Phillip Bay and Western Port where an extensive record of the occurrence and toxicity of Pseudo-nitzschia exists extending from 1987 till the present. Where other regions are involved, due to the variability in the toxicity of the various forms of Pseudo-nitzschia, it would be prudent to follow the more conservative threshold levels proposed in the Australian Victorian Marine Biotoxin Management Plan for Shellfish Farming (2001) or those in other biotoxin management plans, which may be accessed through various websites.
This would also be the case if scallops were harvested rather than mussels, as some evidence exists suggesting that scallops are more likely to accumulate domoic acid than mussels. It is noted that domoic acid is known to bioaccumulate i.e. levels build up in organisms though food chains.
REFERENCES
Arnott, G.H., Reilly, D.J. and Werner, G.F. (1999). Victorian Shellfish Quality Assurance Program. 7. Sanitary Survey Update: Clifton Springs and Grassy Point (port Arlington) Aquaculture Zones. Marine and Freshwater Resources Institute, Report No. 13, 1 – 36
Chang, F.H. (2004). A Review of the Currently Monitored Toxigenic Phytoplankton Species in New Zealand. Report Prepared for New Zealand Food Safety Authority by National Institute of Water & Atmosphere Pty Ltd (NIWA). Report WLG2004-80.
Todd, K. (2001). Australian Victorian Marine Biotoxin Management Plan for Shellfish Farming., Prepared for the Australian Shellfish Quality Assurance Committee (ASQAAC) by the Cawthron Institute. Cawthron Report No. 645
Hallegraeff, G.M. (1994). Species of the Diatom Genus Psuedo-nitzschiain Australian Waters. Botanica Marina, 37: 397 - 411.
Hallegraeff, G. (1997). Algal toxins in Australian Shellfish. In: Foodborne Microorganisms of Public Health Significance. Fifth Edition. AIFST (NSW Branch), Food Microbiology Group.
Hallegraeff, G. (2002). Aquaculturists' Guide to Harmful Australian Microalgae. The Print Centre, Hobart.
Lapworth, C., Hallegraeff, G and Ajani, P.A. (2001). "Identification of Domoic-Acid-producing Pseudo-nitzschiaspecies in Australian Waters" In: Harmful Algal Blooms 2000. Hallegraeff, G.M., Blackburn, S.I., Bolch, C.J. and Lewis, R.J. (Eds). Intergovernmental Oceanographic Commission of UNESCO.
Rhodes, L. (2005). Response to a Review of the Currently Monitored Toxigenic Phytoplankton Species in New Zealand. Cawthron report No. 979: 1-18
Rhizosolenia cf chunii
BACKGROUND
Rhizosolenia cf chunii is straight, cylindrical diatom, often found in chains.
Blooms of this species can impart a bitter taste to mussels and other shellfish and render them unfit for human consumption. The chemical nature of the bitter taste is unknown but the effect can persist for up to 7 months (Hallegraeff, 2002). In the 1987 Port Phillip Bay bloom, the digestive glands of exposed shellfish showed degeneration and significant mortality occurred 3 – 8 months after the bloom (Parry et al, 1989; Hallegraeff, 2002). Consequently, although posing no threat of toxicity to humans, the occurrence of blooms of this species constitutes a major threat to the mussel industry.
MANAGEMENT PROTOCOL
The following management protocol is designed to give growers warning of blooms of this species to facilitate their management of their mussel resource. This potentially includes the transportation of mussels from impacted areas (not currently possible) to areas with lower numbers of Rhizosolenia cf chunii, the suspension of harvesting and the withdrawal of affected mussels from the market. It is based on phytoplankton monitoring and the following information:
- Rhizosolenia cf chunii is regularly detected within the Port Phillip Bay harvesting areas.
- In 1987, this species was responsible for making mussels unpalatable by imparting a bitter taste to them.
- Since then, other instances of this have been recorded by monitoring under the previous Victorian Shellfish Quality Assurance Program (VSQAP).
- The marketing of mussels with bitter taste imparted by R. cf chunii would be deleterious to the aquaculture mussel industry.
This management protocol has been used successfully within the VSQAP from August 1999 to December 2008. It has been adopted for the current Victorian Marine Biotoxin Management Plan (VMBMP) including the mussel harvesting areas in Port Phillip Bay and Western Port.
- Phytoplankton monitoring occurs routinely under the VMBMP.
- Growers are to be kept updated regularly as to the abundance of R. chunii at each harvesting area as each routine sampling event is carried out.
- If Rhizosolenia cf chunii is detected in numbers greater than 10,000 cells/L, a level one warning is to be issued to harvesters, advising that its abundance is rising.
- If Rhizosolenia cf chunii is detected in numbers greater than 20,000 cells/L, a level two warning is to be issued to harvesters that its abundance is approaching levels at which a bitter taste appears in mussels.
- Additional sampling/monitoring may be undertaken by harvesters at any time.
- Harvesters are to be informed once the threat has passed.
VMBMP Phytoplankton Abundance Threshold Levels (cells/L)
Phytoplankton Species | Toxin | Warning Issued to Growers | Tissue Testing | Harvest Suspension (Pending Toxin Analysis) | Harvest Resumption |
Rhizosolenia cf chunii | Non-toxic Bitter Taste | 10,000 Level 1 Warning 20,000 Level 2 Warning | N/A | N/A | N/A |
Harvesting suspension is based on the presence of the bitter taste and is invoked voluntarily by harvesters as they see fit. The role of the VMBMP in relation to R. chunii is to keep growers informed concerning the presence of this species.
REFERENCES
Hallegraeff, G. (2002). Aquaculturists' Guide to Harmful Australian Microalgae. The Print Centre, Hobart.
Parry, G.D., Langdon, J.S. and Huisman, J.M. (1989). Toxic Effects of a Bloom of the Diatom Rhizolsolenia chunii on Shellfish in Port Phillip Bay, South-eastern Australia. Mar. Biol., 102:25 - 41
For further information on this report, contact:
| Name: | Dr Terry Walker |
| Title: | Principal Environmental Consultant |
| Address: |
PO Box 34, New Town, Tasmania 7008 79 Augusta Rd, Lenah Valley, Tasmania 7008 |
| Phone: | 03 6228 6769 |
| Mobile: | 0409 863 590 |
| E-mail: | twalker@ecowise.com.au |
This document has been prepared for the Client named above and is to be used only for the purposes for which it was commissioned. No warranty is given as to its suitability for any other purpose. No responsibility is taken for material provided by Fisheries Victoria.
Ecowise Australia Pty Ltd - ABN 68 074 205 780
