High Value Aquaculture
Our Rural Landscape: Sustainable development through innovation
Final Report ORL 2.3
CMI 100487
ISBN 978-1-74199-510-7
Executive Summary
The application of advanced genetic technologies has the potential to enhance profitability and sustainability of inland aquaculture industries and to facilitate the conservation of native fish biodiversity in Victoria. Native fish species with high commercial, recreation and conservation value have enormous 'untapped' potential for productivity gains through genetic enhancement and reproduction technologies.
This project was designed to utilise innovative genetic and reproduction technologies to create elite strains for aquaculture of a high value inland fish species, in this case Murray cod, and to ensure the maintenance of wild biodiversity of the species. The objectives of the project are based around three main components, selective breeding (technologies to produce new domesticated strains of Murray cod for aquaculture), controlled reproduction (technologies to enhance production, and provide biosecurity & IP protection), and population genetics (technologies to assist wild fisheries management & conservation).
Sixty nine families of newly hatched Murray cod larvae were obtained to establish a genetically diverse founder population for a selective breeding program. DNA markers (microsatellites) isolated from Murray cod were used for parentage analysis, development of a genetic map, identification of linkage groups and discovery of links (QTL's) between markers and aquaculture traits. DNA markers were also used determine and compare the genetic structure of contemporary and historic (pre 1954) wild Murray cod, from across the Murray-Darling Basin (MDB).
Mature Murray cod (and silver perch) held in recirculating aquaculture system were induced to spawn under control environment conditions to demonstrate "out-ofseason" spawning, and to develop chromosome-set manipulation, hybridisation and cryopreservation techniques.
The project characterised 102 new markers and developed a genetic map for Murray cod. Markers were successfully linked to several important aquaculture traits, including growth, skin colour, fish condition and fat content. Murray cod were spawned out-of-season, and reproduction techniques to enhance production biosecurity and IP protection identified. The population structure of wild Murray cod was elucidated, including the discovery of discrete management units within the MDB.
The project has established a Murray cod founder population and developed a Murray cod marker assisted selection system to a pre-commercialisation stage. Uptake of a selective breeding program by industry will assist the sustainable and profitable development of the Murray cod aquaculture industry. Genetics tools for understanding the genetic structure of wild Murray cod populations will be important in developing genetically sound recovery plans for the species at state and national levels, and will ensure biodiversity. Importantly, this project will serve as a template for similar studies on other significant and valued aquatic species.
The capability established in both the R&D and production sectors of the Victorian aquaculture industry is expected to stimulate further private sector investment, industry, regional development and job opportunities, and to provide new and valuable information to relevant resource managers in the public sector (including DPI, DSE, CMAs etc).
1. Project Background
1.1 Project Identification
| Project title: | High value aquaculture in sustainable rural landscapes |
| Project number: | MIS 05194 (ORL 2.3) |
| Project leader: | Dr Brett A Ingram |
| Contact details: | DPI, Snobs Creek, Private Bag 20, Alexandra, Vic, 3714. |
| Project team: | Helen McPartlan (DPI, Attwood), Ben Hayes (DPI , Attwood), Nick Robinson (DPI, Attwood), Hui King Hi (DPI, Snobs Creek), Jenny Nheu (DPI, Attwood), Hayley Mountford (DPI, Attwood), Meaghan Rourke, (DPI, Attwood), William Bravington (DPI, Snobs Creek) & Peter Boyd (DPI, Snobs Creek) |
1.2 Project Context
Our Rural Landscape (ORL) is a four-year science based program funded as part of the Victorian Government's Innovation Statement, centred on the sustainable development of Victoria's primary industries. The State Government has invested $50 million in the program from 2003 to 2007.
The ORL program aims to increase the value of agriculture per unit of natural resource, balancing economic productivity and social outcomes with resource use efficiency and protection of the environment.
The Australian aquaculture industry faces failure unless two conditions are met:
- High performing strains of fish that meet market needs are available to industry
- Environmental sustainability and impact of the industry can be monitored and controlled.
This project was designed to utilise the latest in innovative genetic and reproduction technologies to create elite strains for aquaculture of a high value inland fish species, in this case Murray cod (Maccullochella peelii peelii) (Mitchell) (Percichthyidae), and to ensure the maintenance of wild biodiversity of the species. The elite fish stock that are produced from the project will open up new opportunities for rural communities in Victoria. The stock will be quality assured and demonstrably superior to wild stock for aquaculture and will eliminate the need to source wild animals for aquaculture.
This project developed and applied novel genetic and reproduction technologies for a high value inland aquaculture species which meets multiple management/market needs. The capability established in both the R&D and production sectors of the Victorian aquaculture industry is expected to stimulate further private sector investment, industry, regional development and job opportunities, and to provide new and valuable information to relevant resource managers in the public sector (including DPI, DSE, CMAs etc).
There is enormous potential for the development and creation of market opportunities for genetically enhanced native fish species. With seven years of industrial scale genetic enhancement of salmon in Norway, a highly efficient US $1.5 billion per year export industry was created. Farmed salmon in Norway now reach market size in half the time, are highly efficient at converting food to muscle and are genetically resistant to several major diseases largely through genetic selection and production programs.
A new aquaculture industry in Victoria of this scale would open unprecedented opportunities for employment. The allied breeding program to enhance natural biodiversity and selection of fish for improved feed conversion efficiency will reduce farm waste, enhance Victoria's clean, green image among overseas distributors and consumers, and create positive environmental, conservation and recreational fishing spin offs. Recreational fishing opportunities will improve in rural Victoria as large and genetically biodiverse populations of native fish stock are re-established.
1.3 Project Rationale
The application of advanced genetic technologies to augment conventional selective breeding programs on an industrial scale in Victoria has the potential to significantly improve the production and environmental performance of various high value, inland finfish species. However, the existing industry is too small to cover the full cost of R&D in this area. Furthermore much of the R&D is deemed to be either public good, particularly in relation to the environmental benefits, and/or driven by the need to manage externalities of different industry sectors which directly or indirectly influence aquaculture in Victoria. It is likely that failure will occur in the short to medium term if attention is not paid to selective breeding. Either way, R&D investment in this project is expected to deliver significant triple bottom line benefits to rural communities through facilitation of profitable and sustainable integrated agri-aquaculture based farm diversification.
Native species with high commercial, recreation and conservation value have enormous 'untapped' potential for productivity gains through genetic enhancement and novel reproduction technologies, given that current practice relies on the use of wild stocks of unknown genetic makeup, and mainly natural (uncontrolled) spawning methods under ambient conditions.
Murray cod Maccullochella peelii peelii (Percichthyidae) was selected as the target species in the present study for several reasons. There is an established aquaculture industry based on hatchery production of juveniles for stock enhancement programs and a developing aquaculture grow-out sector in rural Victoria and other states. There is an existing domestic market where it is recognised for its high value and eating qualities. There is interest in the species in several Asian countries; live juveniles have been exported for grow-out and harvested products have been sold into a number of markets overseas. Murray cod is a highly prized recreational angling species, which is valued for its sport and edibility. Due to a decline in abundance and distribution in the wild, the species was listed in 2003 as Vulnerable under the Commonwealth Environment Protection and Biodiversity Conservation Act 1999.
Application of advanced genetic technologies will not only provide innovative solutions to many of these problems but also will provide for adjunct benefits to the management of native finfish biodiversity, particularly for the larger, commercially valuable species which are declining in the wild and yet continue to be managed for multiple purposes (ie. commercial, recreation, conservation). Importantly, Murray cod has recently been nationally listed as a threatened species, thus further emphasising the imperative to reliably close the breeding loop and use only fully domesticated stock for aquaculture purposes thereby eliminating dependency on wild stocks; a plus for biodiversity.
1.4 Key Stakeholders
Key stakeholders in the selective breeding and controlled reproduction components of the project will be the aquaculture industry of Victoria (hatcheries and growers, along with ancillary industries), especially Murray cod farmers who will be the immediate users of the research findings. In particular, one rural industry group, located in northwest Victoria, has already expressed interest in being involved in the commercialisation of a selective breeding program for Murray cod. However, as Murray cod are also farmed interstate and overseas, these industries will also have a keen interest in outcomes from the project. The Victorian Fisheries Authority (VFA), a co-investor in the project, views the project as supporting industry development in the state.
Key stakeholders, and immediate next users of outputs from the population genetics component of the project are fisheries and conservation managers and policy makers, and other genetics researchers. Since Murray cod is endemic to the Murray-Darling Basin (MDB), stakeholders are represented by fisheries and conservation agencies federally, including the Murray-Darling Basin Commission (MDBC) and DEH, and at the state level (Qld, NSW, SA and VIC). The Victorian Fisheries Authority and DSE are the key stakeholders in Victoria. The recreational fishing industry and especially in rural areas are also stakeholders and stand to benefit from enhanced management of wild populations, including genetically responsible stocking practices. Outputs from the project have already been used by DSE, The Victorian Fisheries Authority and other researchers studying population genetics of related species (Maccullochella and Macquaria).
1.5 Objectives
This project applied innovative genetic and reproduction technologies to enhance profitable and sustainable inland aquaculture industries and to facilitate the conservation of native fish biodiversity in Victoria.
The objectives of the project are based around three main components:
Selective Breeding: To develop genetic technologies to enable the development of new domesticated strains of Murray cod selected for high performance (growth etc.) in aquaculture.
Controlled reproduction: To develop controlled reproduction technologies (including controlled environment spawning, cryopreservation, and chromosome-set manipulation) for Murray cod and other chosen species to enhance production and to provide biosecurity & IP protection.
Population Genetics: To develop genetic technologies and tools for wild fisheries management & conservation of biodiversity, and to manage the production of genetically certified 'wild strain' seedstock where required.
1.6 Methods
1.6.1 Selective breeding
Establishment of founder population
In order to establish a genetically diverse founder population of Murray cod for a selective breeding program, families of newly hatched larvae were obtained from hatcheries based in Queensland (1 hatchery), NSW (3 hatcheries) and Victoria (DPI, Snobs Creek). A prerequisite of these families were that they were derived from broodstock collected from the wild, and that each family represented a single spawning from one pair of fish. Thirty four families, each containing between 6,000 and 13,000 pre-feeding larvae were obtained in October and November 2003. However, a major power failure in holding facilities in December 2004 resulted in significant loss of families and stock. Consequently, during October and November 2005 another 39 families were obtained.
Custom built facilities were established at DPI, Snobs Creek to hold the families. These included additional tanks and troughs in existing facilities to hold larvae and fingerings, and two new 20,000 litre recirculating aquaculture systems (RAS), fitted with 39 fibreglass tanks, and located in a greenhouse structure for holding larger fish. Fish were reared according to standard procedures for growing Murray cod (Ingram and De Silva 2004). Families were maintained separately until fish were large enough to individually tag with microchips (Trovan).
Trait identification
There is limited published information on traits considered favourable for Murray cod producers and retailers. Consequently, five Murray cod hatcheries and 16 retailers at three fish markets in the Melbourne metropolitan were surveyed by interview to identify favourable traits in Murray cod (Brown 2005). Results from this study were then used to guide phenotypic measurements for quantitative trait loci (QTL screening). The survey also identified whom and where the major markets were for Murray cod.
Isolation and characterisation of Murray cod microsatellite markers
DNA was extracted from fin clippings of Murray cod using the QIAGEN Dneasy Tissue Kit. DNA markers (microsatellites) were isolated using an enrichment library approach. Primers for the PCR were designed to incorporate one of four fluorescent tags on one of each pair of primers so that they could be analysed on an ABI 3700 or ABI 3730. The primer pairs were then grouped according to size and tag colour so that they could be optimised into multiplex reactions using the QIAGEN multiplex kit. Initially the markers were tested using a set of 34 un-related animals from the broodstock ponds at DPI, Snobs Creek. The markers were also analysed across a range of related and non-related fish species. The results from these analyses can be found in Rourke et al. (2007).
Production of a genetic map of Murray cod
Four families were selected that showed no evidence of having resulted from a polygonous spawning event. At three weeks of age, 10,000 larvae from each family were mixed at and grown in a fry pond at DPI, Snobs Creek. At 14 weeks of age 9,340 of these fish were transported to Deakin University, Warrnambool (DUW) to be on-grown in a RAS under commercial conditions. The remaining fish were kept at DPI Snobs creek as a back-up. At 35 weeks of age 2,290 fish at DUW were microchipped (Trovan) for individual identification. After selecting and tagging 500 of the largest fish (mean Wt 119g) and 500 of the smallest fish (mean Wt 32 g), the balance were randomly selected from three size classes (mean Wts: 50g, 71g, and 97g). At the same time the total length (TL) and weight (Wt) were recorded and a duplicate fin clipping were taken from each fish and placed into 96 well collection racks, which were then stored at -20C. The remaining non-tagged fish were removed from the experiment. At 45 weeks of age, the TL and Wt of all fish were measured, and fish that had shed microchips were implanted with a new microchip and fin-clipped for family assignment.
One of each collection rack was extracted, as described above. Each of 112 microsatellite markers were analysed for the first 28 plates. A one pass method of analysis was performed only. The data was analysed using Genemapper after which it was exported into a genotyping database. Parentage was assigned using approximately 7 DNA markers. A parentage check was then performed with every marker. The data was re-checked for animals found to have pedigree errors and any animals with more than four pedigree errors were removed from the data. The data from 1850 individuals was exported to develop a genetic map and to identify linkage groups for the microsatellite markers.
Quantitative Trait Loci (QTL) analysis
At 63 weeks of age, all surviving micro-chipped fish at DUW were measured, which included TL, Wt, skin colour (tristimulus values using a Minolta CR-200 light meter), fat content (Distell Fish Fat meter, model FM-692) and physical deformities. Fish condition was calculated from the TL and Wt data. Specific growth rates (SGR) was calculated for each individual using Wt data from each sampling period. The L* tristimulus value was used to represent "lightness" while chroma and hue were calculated from the a* and b* tristimulus values (Robb 2001). These data, together with linkage group data, were analysed using QTL express to determine if there are QTL present for the measured traits.
Data were analysed for two of the fish families that survived the power outage at Snobs Creek. As the fish were exposed to higher temperatures and lower oxygen levels it was decided that any survivors could be genetically able to cope with stress. If this was the case and the genes involved in stress management were close to the DNA markers tested it would be supposed that there would be a skewing of inheritance of alleles. The data set was analysed to determine whether any of the alleles were at a higher or lower level than what would be expected by random chance (knowing the parental alleles). This was calculated by use of a Chi Square test.
1.6.2 Controlled reproduction
Controlled spawning
Two hundred and twenty non-selected maturing and mature 1st generation Murray cod that had been reared in captivity since birth were placed into a 22,000L RAS at DPI, Snobs Creek, where research into the controlled reproduction of Murray cod is being conducted on a group of broodstock held in a recirculating aquaculture system under artificial conditions. Fifty mature 1st generation silver perch, Bidyanus bidyanus (Mitchell) (Terapontidae) another endemic aquaculture species that were obtained from NSW department of Primaries Industries, were also placed into the RAS to provide a second species for controlled breeding and chromosome-set manipulation trials.
Photoperiod was managed by a Cfish Photoperiod Controller (Kingston Electronics, Tas) which provided control over daylength, twilight and season duration for any given latitude and longitude. Water temperature was controlled manually by a heat exchanger heated from a gas fired boiler and thermostat-controlled electric chiller. A photo-thermal regime obtained from Euston (on the Murray River) was used as a standard. In order to undertake controlled environment spawning, this standard regime was advanced by 2 months by "shrinking" a normal annual season. This also provided the opportunity to undertake interspecific hybridisation trials with trout cod Maccullochella macquariensis (Cuvier), a closely related species to Murray cod that spawns earlier in the season.
Fish were fed on commercially available fish pellets through the study, but in latter years the diet of Murray cod was supplemented with fresh pieces of trout. Spawning was induced by hormone injection and hand stripping of gametes following ovulation as described in Rowland (1986) and Ingram and Rimmer (1992) for Murray cod, and Rowland (1984) for silver perch. Egg incubation, hatching and larviculture techniques followed standard hatchery procedures for these species.
Chromosome-set manipulation and hybridisation
An important aspect of the project involved identifying options for producing sexually benign (sterile or infertile) progeny. While such research is important in terms of protecting intellectual property and affording enhanced performance in aquaculture, production of sterile stock will also eliminate the reproductive potential of progeny so that, in the event of escape, the potential risk of a genetic impact on wild stocks is minimised. Chromosome-set manipulation involving triploidy, and inter-specific hybridisation were investigated to explore these options.
Triploidy induction trials were conducted on newly fertilised eggs of Murray cod and silver perch from controlled spawning trials. Batches of eggs from each spawning were exposed to different temperature (cold and heat) and pressure shocks for different lengths of time. A batch of eggs from each spawning was treated normally to serve as a control. Following shock treatment eggs were incubated separately and after hatching, hatch rates in treatments were compared against hatch rates of control groups. The level of triploidy in each batch of larvae in each treatment was determined by counting chromosomes at 1000x magnification following treatment of live fish with Colchacine and staining smears of the treated emulsified fish on microscope slides with Giemsa stain (Kligerman and Bloom 1977).
Hybridisation between Murray cod trout cod, though rare, occurs naturally in the Murray River where both species occur sympatrically, and has also been induced in captive fish previously (Douglas et al. 1995). Excess sperm from trout cod broodstock held at DPI, Snobs Creek for production of juveniles for restocking was used to fertilise the eggs of Murray cod. A small number of eggs from trout cod were fertilised with Murray cod sperm to determine hatch rates in reciprocal crosses only. Spawning was induced by hormone induction (Rowland 1986, Ingram and Rimmer 1992) and egg incubation, hatching and larviculture techniques followed standard hatchery procedures for these species. After hatching, hatch rates in treatments were compared against control hatch rates (pure trout cod and pure Murray cod spawnings). Evaluation of the performance of hybrids was conducted in two experiments. In one experiment, Murray cod and hybrids were implanted with coded-wire tags (Northwest Marine Technology, USA) and communally reared at DPI, Snobs Creek. In a second experiment, approx 2,000 hybrids were transferred to a biosecure commercial Murray cod RAS for aquaculture performance (ie hybrid vigour such as increased growth and environmental tolerance) and marketing value under commercial conditions.
Sperm cryopreservation
During the 2004, 2005 and 2006 breeding season a series of experiments were conducted to identify methods for cryopreserving Murray cod and trout cod sperm. Trials were conducted in collaboration with the Centre for Reproduction and Development, Monash Institute of Medical Research (Clayton, Vic), the Department of Physiology, Monash University (Clayton, Vic) and CryoLogic Pty. Ltd (Mulgrave, Vic.).
Trials were conducted on pooled samples of fresh sperm from running ripe males held in a controlled environment RAS, or broodstock ponds at DPI, Snobs Creek. Trials tested a range of cryopreservation extenders in various combinations and concentrations, and freezing methods. Freezing was conducted in either a Freeze-Control® Cryochamber (Cryologics, Australia) connected to a temperature controller, in liquid nitrogen vapour or on dry ice. Frozen sperm was stored in liquid nitrogen until thawing. Sperm osmolarity was measured in an Osmomat 030 cryoscopic osmometer. Sperm activity (percent active and motility), pre- and post thaw, was subjectively assessed by light microscopy, and recorded on video or DVD for computer assisted sperm assessment (CASA) using an Integrated Visual Optical System (IVOS) Sperm Analyzer (Hamilton Thorne Biosciences, USA).
Preliminary fertilization trials used thawed sperm were conducted in 2006. Replicate batches of eggs from individual females were fertilised with either fresh or thawed sperm and incubated separately.
1.6.3 Population genetics
Parentage analysis of captive broodstock
Parentage testing of 46 captive bred Murray cod families (collected from natural spawnings in ponds at DPI, Snobs Creek) was carried out to determine mating habits. Fin-clip samples were taken for DNA extraction from the entire broodstock population (78 fish) prior to the 2005 breeding spawning season. Samples were stored in 90% ethanol until DNA extraction. Over three spawning seasons (2003, 204 and 2005) every batch of eggs collected from 11 broodstock ponds during the spawning season were removed to the hatchery and incubated separately until hatching. The number of eggs and hatched larvae in each spawning were determined by volumetric displacement and electronic counter, respectively. Approximately thirty larvae from every spawning were collected for DNA extraction and parentage assignment.
DNA was extracted from both the broodstock and larvae samples using the QIAGEN Dneasy Tissue Kit. Nine microsatellite loci described in the present study (Rourke et al. 2007), or by Loughnan et al. (2004) were selected based on high heterozygosity, ease of amplification, ease of scoring and ability to be combined into one of two multiplex PCR reactions. Multiplex reactions were performed using the commercially available kit from QIAGEN. Summary genetic diversity statistics, including the number of alleles (a) allelic richness (ar) and expected heterozygosities (He), were calculated for the 78 broodstock, progeny from each season and progeny pooled over all seasons, using FSTAT 2.9.3 (Goudet 1995, http://www2.unil.ch/popgen/softwares/fstat.htm). Permutation tests (15000 permutations) were performed in FSTAT. Exact tests for deviation from Hardy-Weinberg expectations, and linkage disequilibrium, were performed in Arlequin 3.1 (Schneider et al. 2000, http://cmpg.unibe.ch/software/arlequin3/). Probability of parentage exclusion values were calculated for each individual locus and for the nine loci combined in CERVUS (Marshall et al. 1998) and parentage was assigned to each individual also using CERVUS. Following assignment of parents to each spawning, the number of progeny surviving to hatch was calculated for each male and female parent, allowing the number of effective males and females to be estimated.
Contemporary population structure
In order to determine the genetic structure of contemporary wild Murray cod, tissue samples (finclips and fish scales) were obtained from 700 fish from 19 river catchments across the Murray-Darling Basin (MDB). These included samples collected as part of an earlier study (Bearlin and Tikel 2003). Sample collection in the present study was assisted by state fisheries agencies: DSE, New South Wales Department of Primary Industries, Queensland Department of Primary Industries and Fisheries and Primary Industries and Resources South Australia (PIRSA). Recreational angling groups have also helped with the collection of samples. Funding from the Holsworth Wildlife Research Fund and the NSW Recreational Fishing Trust also supported sample collection and analysis.
Samples were either stored in 100% ethanol (finclips), or allowed to dry in paper envelopes (scales). DNA was extracted from fin-clips and approximately 6-10 scales (depending on size) using the DNeasy 96 Tissue kit (QIAGEN) following the manufacturer's standard protocol. Seventeen microsatellite loci were selected for population analysis based on their small fragment size (most <200bp), because they were also to be used on the potentially degraded DNA from the historical samples.
Historical population structure
An historical collection of Murray cod scales, originally collected for fish aging purposes, was located at DPI, Snobs Creek. DNA from 6-12 scales of 361 fish collected from six southern river catchments between 1948 and 1953, was extracted by using the QIAGEN DNeasy Kit and decreasing solution volumes to increase DNA yields. Multiplex PCR reactions were carried out as for the contemporary samples and every historical sample was amplified in at least two separate reactions per locus to ensure accuracy of results. Genotypes were only included in analyses if they were based on identical results from two independent reactions.
Historical samples (pre 1954) were compared against contemporary samples (post 1995) to identify temporal changes in the genetic structure of Murray cod in the southern part of the MDB, and to identify potential impacts of stock enhance programs, which commenced in the early 1980's.
2. Results and Discussion
2.1 Outputs
2.1.1 Selective Breeding
Founder population
Currently, approximately 4,600 fish from 40 genetically diverse families of Murray cod are being maintained. Most fish are held in facilities at DPI, Snobs Creek, but some are held at Deakin University, Warrnambool (100 fish) and Thurla Farms, Red cliffs (900 fish) for risk management and QTL screening purposes. These fish, once mature will provide a genepool from which new broodstock possessing traits of interest (as identified by QTL screening) can be selected for breeding new domesticated strains of Murray cod selected for high performance (growth etc.) in aquaculture. Stock that were obtained in 2003 are now reaching maturity with some fish approaching 5 kg in weight. These fish may be mature and ready to spawn for the first time in November 2007.
Trait identification
Murray cod hatcheries indicated that survival traits including stress resistance, hardiness and disease resistance were important for production. The retailers believed Murray cod was a high value, speciality fish in the market. Important physical traits for selling Murray cod were skin colour (30%) and flesh texture (31%), followed by fish size (23%). Lighter coloured fish, typical of those caught from the wild, were preferred over darker coloured fish. A total of 37% of retailers preferred Murray cod >2kg while 27% preferred 1-2 kg fish. Some retailers commented on the lack of quality of these traits in the fish they received from producers. Retailers also indicated there was demand for Murray cod in the market and that sales would increase if there was a reliable and regular supply of quality Murray cod. Further details of this survey are provided in Brown (2005).
Based on these results, as well as review of literature, potential favourable traits of interest for Murray cod aquaculture are:
- Production traits: Growth (weight and length), condition, food conversion efficiency, survival (hardiness and stress tolerance), disease resistance and weanability.
- Reproduction traits: Time to maturity and sex.
- Market traits: Size, appearance (skin colour, flesh colour, and flesh texture) and taste.
Isolation and characterisation of Murray cod microsatellite markers
One hundred and three microsatellites successfully amplified in Murray cod, of which 101 were polymorphic and two were monomorphic. Excluding the monomorphic loci, the number of alleles per locus ranged from two to 19 alleles per locus (mean seven alleles per locus). The characteristics of the new primers are provided in Rourke et al. (2007). Expected heterozygosities ranged from 0.066 to 0.95 and the average expected heterozygosity was 0.64. All loci were in Hardy-Weinberg equilibrium (GENEPOP, Raymond and Rousset 1995) except for seven which had a deficiency of heterozygotes, possibly due to null alleles. Tests for linkage disequilibrium (GENEPOP) were carried out on the original 34 unrelated animals, and 193 pairs of loci showed significant linkage disequilibrium from more than 5000 possible pairwise locus combinations.
These new loci have been used for the identification of quantitative trait loci to improve the productivity of cultured Murray cod, and to assess wild and hatchery stock structure of Murray cod for management purposes.
Production of a genetic map of Murray cod
The Murray cod genetic map is currently being developed and analysed for the four communally reared Murray cod families. So far, 20 linkage groups, with between two and seven markers per group, have been identified. In additional, large differences in recombination rates between males & females have also been identified. The implications of this finding to selective breeding in Murray cod are yet to be determined.
Quantitative Trait Loci (QTL) analysis
Measurement of fish from the four families combined at 3 weeks of age showed that a number of important traits were quite variable within and between families. Fish from one family in particular (Sp5) grew faster (Figure 1) and more survived than in the other three families. At 63 weeks of age, weight ranged from 57g to 886g.
A subset of microsatellite markers have been successfully linked to a number of important aquaculture traits, including growth (weight at measurement and specific growth rate), skin colour, fish condition and carcass fat content (Figure 1). However, analyses performed on the surviving fish found no evidence of any of the DNA markers being inherited with the "survival" trait. Nevertheless, this information can now be used to select broodstock with these traits to produce new, high performing strains of fish for the aquaculture industry.
These preliminary results indicate strong potential for genetic selection of key aquaculture traits in a domesticated breeding program for Murray cod. Now that microsatellite markers have been linked to favourable traits, a "proof-of-concept" project will need to be undertaken to evaluate the performance
of selectively bred strains of Murray cod.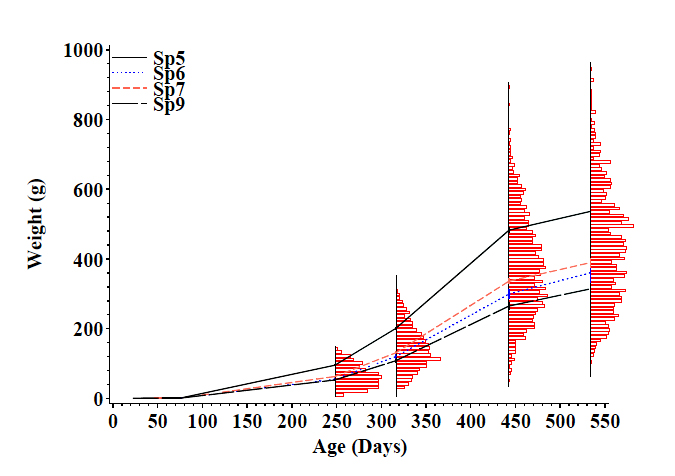

Figure 1. Variation in (a) Growth (lines = mean values and frequency distribution of weight at four sampling dates = bars), (b) condition, an indicator "fitness" or "well-being", and (c) colour, of four communally reared Murray cod families
2.1.2 Controlled reproduction
Controlled spawning
Broodstock held in a RAS under an artificial photo-thermal regime were successfully induced to spawn outside their normal breeding season by manipulating the temperature and light regimes in the recirculating aquaculture system and hormone-induction of ovulation. In 2004 when the first spawning trails were conducted, 90% of fish injected with hormone ovulated and were stripped, but hatch rates averaged 3%, and were substantially lower than hatch rates observed in natural pond spawnings at DPI, Snobs Creek (mean 50%). However by the third year of trials hatch rates of up to 67% were obtained. Silver perch broodstock also held in the RAS were also successfully induced to spawn "out-of-season" using the same methods.
Controlled breeding of these species will increases the flexibility of selective breeding programs and enable greater control over fish matings and seedstock production.
Chromosome-set manipulation and hybridisation
Triploidy was induced in both Murray cod and silver perch by shocking eggs shortly after fertilisation. Murray cod eggs in some shock treatments failed to hatch while in other treatments hatch rates ranged from <1% to >100% of control (non-shocked) hatch rates. Heat and cold shocks failed to induce triploidy in Murray cod, whereas some pressure shocks induced various levels triploidy (up to 100% in some replicates). In silver perch, hatch rates were highly variable and heat shock induced triploidy, but the level of induction was low (approx. 33%). Experiments will need to be conducted to refine these techniques and assess the aquaculture performance of triploids.
Hybridisation was successfully induced in both direct and reciprocal crosses between Murray cod and trout cod. Hatch rates of maternal Murray cod and maternal trout cod hybrids were on average 155% and 49%, respectively, of hatch rates in control groups (pure strain spawnings). Preliminary results from culture trials indicate that hybrids have inferior growth rates and tend to be more aggressive than Murray cod. The marketability and fertility of hybrids will be assessed once fish reach a harvestable size and maturity.
Sperm cryopreservation
Murray cod sperm are 27-30 μm in length with a spherical to kidney-shaped head approximately 1.3 μm across and no obvious mid-piece. The tail is a single, filamentous, flagellum. Trials identified cryoprotectant composition and freezing methods for Murray cod sperm that provided up to 20% sperm motility post thawing. Preliminary fertilisation trials resulted in a successful hatching of eggs fertilised with thawed sperm, but hatch rate was low (4%). Further trials are required to optimise freezing, thawing and fertilisation procedures.
Cryopreserving sperm will provide a cost-effective means of storing and preserving genetically unique material indefinitely for conservation and selective breeding programs. Storage of genetic material in gamete banks (gene banking) is an important tool in the management of biodiversity for conservation purposes by ensuring that diversity is not lost from the gene pool.
2.1.3 Population genetics
Parentage analysis of captive broodstock
Murray cod are generally believed to monogamous. However, parentage analysis of captive Murray cod revealed that broodstock are often polygynous rather than exclusively monogamous. Eight separate polygynous spawnings (males fertilising the eggs of two females) were detected among 46 spawnings. Polygynous spawnings were distributed across five separate ponds, suggesting that this was not an isolated event promoted by conditions unique to a particular pond. The female with the smaller contribution of eggs in each polygynous spawning was responsible for 3 to 30% of the total number of eggs. It is possible that these females contributed a much smaller percentage of the eggs because they had previously spawned in another nest. The results of the parentage analysis confirmed that captive Murray cod, of both sexes, are capable of multiple spawning events per season. It is evident from the current genetic data that approximately half the broodstock did not reproduce over the three year period. These results have substantial implications for genetic management of captive broodstock used in stock enhancement programs. Management practices need take these findings into account in order to optimise genetic diversity in released fish.
Contemporary population structure
Analysis of the contemporary genetic population structure of Murray cod across the MDB has revealed up to six populations. Fish from the Lachlan and Macquarie-Bogan River catchments each represent a discrete genetic cluster, while fish from the Border, Namoi and Gwydir River catchments show a slightly
lower degree of differentiation and evidence of restricted gene flow. Fish from other MDB catchments are apparently one large panmictic population (Figure 2). These results suggest that that populations in rivers flowing into the Murray River are highly connected, while those terminating in swamps and
wetlands, such as the Great Cumbung swamp (Lachlan catchment) and the Macquarie marches (Macquarie catchment), are historically isolated.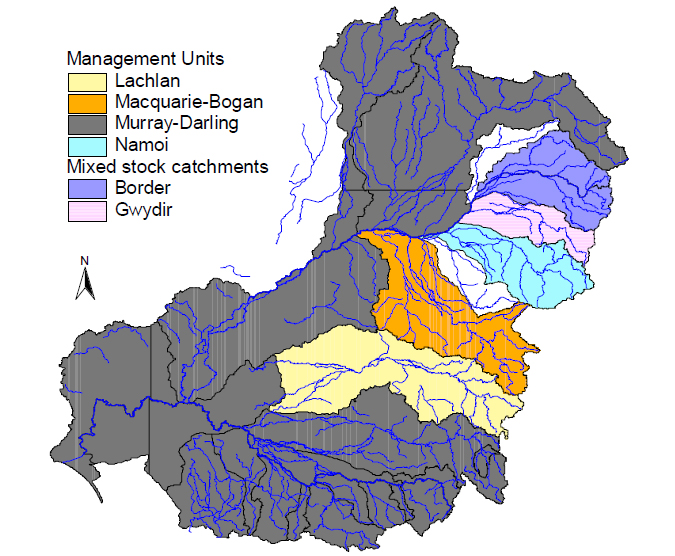
Figure 2. Potentially unique populations (management units) of Murray cod in the Murray-Darling basin
Results of this study suggest that stock enhancement practices have had a mixed effect on the Murray cod populations in the MDB. Most catchment populations, including those in the southern and western MDB, are genetically panmictic. The two most distinctive populations, the Lachlan and Macquarie-Bogan, have retained their genetic uniqueness despite extensive stocking of fish into large impoundments within these catchments. These manmade barriers may have prevented stocked fish from mixing with native strains in the rivers downstream.
This is important information for the long-term management of the genetic diversity of wild populations. For example, managing and optimising the genetic diversity of captivity-bred progeny used in stock enhancement programs will require a clear understanding of the breeding success of all broodstock, which can be affected by mating patterns, and of the receiving populations. More simply, it may be necessary to regularly replace broodstock with new stock and to obtain broodstock from further afield in order to maintain the required level of genetic diversity. Currently, researchers are using data collected from this study to develop a model to evaluate the effects of different breeding and stocking practices on the genetic diversity of wild populations. This model will assist fisheries managers to implement genetically sound stock enhancement programs.
Historical population structure
Temporal comparisons between the historical samples (1948-1953) and contemporary samples (post 1995) from five southern MDB catchments has demonstrated limited genetic differentiation, and therefore no change in either genetic diversity or structure in these catchments over the past half centaury. Analyses found no evidence for recent [2Ne-4Ne generations (Piry et al 1999)] bottlenecks in the southern MDB populations preceding either sampling time point, and that these populations were genetically homogeneous, which is consistent with the biogeographic connectivity between catchments and the migratory ability of Murray cod.
Results indicate that there has been little genetic impact from Murray cod stock enhancement programs in these areas. Stocking activities have had a virtually undetectable genetic effect on these populations, which is probably due to broodstock being obtained primarily within these southern catchments and continual replacement of broodstock with fresh stock from the wild. Low contemporary effective population size contrasted with lack of recent detectable population bottlenecks or loss of genetic diversity and is probably a reflection of life-history traits such as long lifespan and long generation time, masking any genetic effects of population decline.
2.2 Unexpected Outputs
Cross-amplification of the new loci developed for Murray cod was tested on 19 other species of fish (Figure 3). Congeners amplified more reliably than any other species. Within the Percichthyidae, 79-94% of loci crossed-amplified in three other Maccullochella species and 18-38% four Macquaria species (Rourke et al. 2007). These results make this set of microsatellite markers an extremely useful resource for future genetic studies of percichthyids. Indeed, these markers have already been used in population genetics
studies of the endangered trout cod (M. macquariensis) (DSE/MDBC) and eastern freshwater cod (M. ikei) (Southern Cross University), and to distinguish Macquaria spp. and their hybrids (The Victorian Fisheries Authority). Of the other species tested, successful amplification
for at least one locus was achieved in all but three species (Rourke et al. 2007).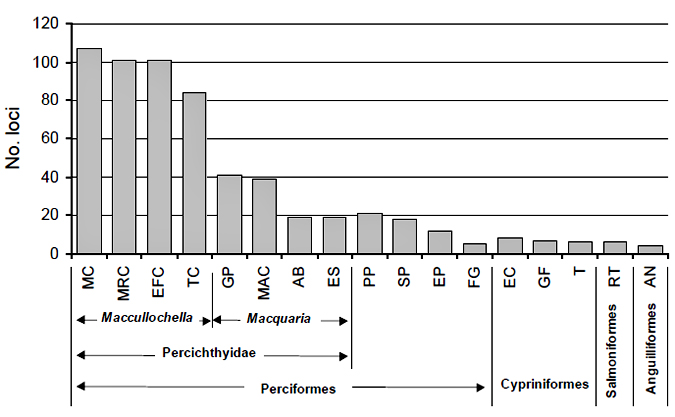
Figure 3. Cross-amplification of Murray cod microsatellite markers in other species of fish (after Rourke et al. 2007)
Early detection of sex is important in evaluating reproduction technologies that affect or manipulate sex. However, outside the breeding season Murray cod cannot be accurately sexed without applying invasive techniques whilst juveniles cannot be sexed at all. During 2007, an Honours student from RMIT was engaged to investigate options for determining sex of Murray cod. This study has identified a non-destructive method, which involves detecting the presence of vitellogenin (yolk protein) in blood plasma, which reliably identifies maturing and mature females. This important tool has immediate application in assessment of wild Murray cod stocks by increasing critical biological and population data on the species, such as sex ratios and size at maturity, which will assist fisheries management. Further studies will refine the technique (including use of mucus instead of blood plasma) and will determine if it can be applied to other percichthyids of commercial, recreational and conservation value.
2.3 Significance of Outputs
This is the first time that an expansive genetic study has been conducted on the Murray cod. This study developed 102 new genetic markers which were subsequently built into the first Murray cod genetic map. This program also established lines of Murray cod that showed variation in production levels and in another first these data are now being analysed with the genetic data to determine whether quantitative trait loci can be detected. The early results from this are promising.
This study has showed that Murray cod and silver perch broodstock can be successfully spawned "out-of-season" under controlled environment conditions, and that triploidy can be induced in both species. Murray cod were also successfully crossed with a closely related with to evaluate of hybrid vigour and sterility in progeny.
Techniques to cryospreserve sperm for gamete and gene management purposes were also developed. A simple protein test has been applied to the Murray cod distinguishes sexually maturing females from males and immature females. This is the first time these technologies have been demonstrated in these species.
A subset of the genetic markers was used to perform a thorough genetic analysis of current and historical Murray cod samples from the Murray Darling Basin. The data has shown that some populations are unique and need to be carefully managed. Comparison between historical samples (1948-1963) and contemporary samples (post 1995) from southern catchments has demonstrated limited genetic differentiation, suggesting that re-stocking has not had an effect on the natural fish populations. Genetic analyses of Murray cod broodstock has provided insights into the previously unknown breeding habits of this species. Contrary to the belief that they form monogamous pairs and breed one a year, occasionally polygamy spawnings occur and some fish spawn twice in a breeding season.
3. Achievements and Outcomes
3.1 Dissemination and Extension
The Murray cod project has had a high profile and there were numerous media presentations (radio, newspaper, media releases from the Minister). Engagement with a wide variety of groups was achieved during the project.
Several universities have been involved in the program through collaborative projects and joint supervision of PhD and honours students. These included Monash University, Flinders University, La Trobe University, Melbourne University, RMIT, Deakin University and the Monash Institute of Medical Research.
Industry has been engaged by the project through on-farm evaluations trials (Warrnambool Trout Farm, Warrnambool and Thurla Farms, Red Cliffs). The developing Murray cod farming industry located in north-western Victoria has already expressed interest in collaborating on the commercialisation of a Murray cod selective breeding program.
Fisheries researchers from other agencies, both within Victoria (DSE) and from other states (DPI-Qld, DPI-NSW, SARDI and MDFRC) were engaged from the outset of the project to assist in collection of samples of wild Murray cod from across the Murray-Darling Basin. Recreational angling clubs and individual anglers also assisted in collecting samples.
A scientist was hosted from Network of Aquaculture Centres in Asia-Pacific (NACA) (Dr Thuy Nguyen), who gained new skills in the area of fish molecular genetics, with the resulting data being published in a joint publication. Training in fish reproduction and larviculture was also provided to technicians from the Research Institute for Aquaculture No.1, Vietnam (Mr Le Quang Hung & Mr Chien) and the Department of Agriculture, Sarawak, Malaysia (Mr David Tinggi). On-going links have been further developed with these groups.
Staff from this project have taken part in national and international workshops and symposia on fish biology and genetics, and several national aquaculture industry conferences. The project team have contributed to 13 conference presentations, seven technical reports, one book chapter and two refereed journal publications. Another 10 journal manuscripts are in preparation. Information on the results of the research were provided to other research groups within and outside DPI through ad hoc workshops, meetings and seminars.
3.2 Next User Reactions
The Murray cod farming industry in Victoria has shown strong and positive reaction to the potential of a selective breeding program for Murray cod. This was expressed most recently at the Our Rural Landscape - Seminars & visit Sunraysia aquaculture sites (Mildura, 13 December 2006). Since the commencement of the project, enquiries in relation to Murray cod farming have increased as the aquaculture value of the species has grown. Direct enquiries have been received from commercial aquaculture businesses in China, Taiwan, the United Kingdom and indirectly (through Australian fingerling suppliers) from Hong Kong.
Aquaculture industries in other states have shown interest in the development of Murray cod aquaculture. In particular, the Aquaculture Association of Queensland have invited the Project Leader to attend the 2007 Annual conference (August 2007) as the keynote speaker to present findings from this project (and ORL 1.3).
3.3 Changes in Knowledge, Attitudes, Skills and Aspirations
Industry interest in accessing selectively bred Murray cod has grown since the commencement of the project. Most recently, and in association with ORL 1.3 project staff and AVS, several meetings have occurred to discuss investment in commercialisation of a selective breeding program. Another industry collaborator (involved in on-farm evaluation trials) has expressed interest in participating in further collaborative R&D in this area. The Victorian Fisheries Authority has also supported this process.
The national Murray cod recovery plan steering committee has requested information from the project. Information on the genetic structure of Murray cod from this project will be important in formulating recovery and management plans for different populations. Under an AVS agreement, markers identified during the project were used to study the genetic structure of eastern freshwater cod (Maccullochella ikei) by Southern Cross University. DSE is now funding a study that is evaluating the genetic structure of a population of endangered trout cod established from re-stocking.
3.4 Practice Change
These preliminary results from the project indicate strong potential for genetic selection of key aquaculture traits in a domesticated breeding program for Murray cod. Now that microsatellite markers have been linked to favourable traits, a "proof-ofconcept" phase will need to be undertaken to evaluate the performance of selectively bred strains of Murray cod. This phase would ideally be undertaken in collaboration with industry under a joint venture agreement.
The Victorian Fisheries Authority has accepted DNA technologies as a useful tool for helping them establish or verify their brood stocks. The Victorian Fisheries Authority has invested in the use of these technologies for the screening of Australian Bass prior to release to ensure that they are purebred and not hybrids with Estuary Perch. Project staff also contributed to the identification and ranking of genetic risks associated with translocations in Victorian inland aquaculture and non-commercial private waters.
In 2007/08 Fisheries Victoria commenced funding a new biotechnology project that will feature the development of a genetics-based selective breeding program for mussels (Mytilus edulis). This project is also supported by the Victorian mussel industry which has signed a joint venture agreement with Fisheries Victoria to establish a mussel hatchery at DPI, Queenscliff.
3.5 Achievements and Outcomes
Skills and science capabilities of project staff were greatly increased in the areas of population genetics, molecular genetics, fish QTL analyses and reproduction technologies. Highlights of this achievement included Meaghan Rourke (PhD student) being awarded ORL Young achiever (May 2005) and Brett Ingram (Project Leader) was named one of Victoria's leading scientists in Science Week (13–21 August 2006), which highlighted the following PIRVic scientists as outstanding examples of PIRVic's R&D strength.
There have been a number of significant achievements resulting from this project. These include:
- Identification of traits of interest to the Murray cod industry
- Isolation and characterisation of 102 new microsatellite markers
- Genetic map of Murray cod
- Genetic markers linked to traits of commercial interest
- The refinement of controlled spawning procedures for Murray cod
- The development of reproduction techniques (triploidy and hybridisation) that will enhance production and may provide IP biosecurity and protection.
- A method to cryospreserve Murray cod sperm
- A non-destructive method of identifying female Murray cod
- An understanding of the population structure of naturally occurring contemporary Murray cod populations
- An understanding of the population structure of naturally occurring historical Murray cod populations
- An understanding of the impact of re-stocking on naturally occurring populations
- An understanding of the mating behaviour of Murray cod in breeding ponds.
The project has established a Murray cod founder population and developed a Murray cod marker assisted selection system to a pre-commercialisation stage. Uptake of a selective breeding program by industry will assist the sustainable and profitable development of the Murray cod aquaculture industry and will assist expansion of the industry into new markets (such as through export of new strains of Murray cod fry to overseas aquaculture industries).
Genetics tools for understanding the genetic structure of wild Murray cod populations will be important in developing genetically sound recovery plans for the species at state and national levels, and will ensure biodiversity. These tools will also assist in the management of Murray cod as a valued natural resource in rural Victoria, in particular through development of genetically responsible stocking practices to maintain and enhance recreational fisheries. Importantly, this project will serve as a template for similar studies on other significant and valued aquatic species.
The project had a strong collaboration focus with other researchers from universities university level, other agencies involved in fisheries and aquaculture research within the state, interstate and internationally, and with industry. This collaboration greatly assisted in the effective execution of research and delivery of outputs
There is now recognition of DPI as a leader in aquaculture and fish genetic research for aquaculture and fisheries management and conservation, as indicated by the instigation of new projects that incorporate genetics into aquaculture and fisheries R&D.
3.6 Learnings
In December 2004, a major power failure cased the death of a significant number of fish in the founder populations. This event resulted in a heightened level of awareness and increased the level of staff skills in identifying, assessing and managing risks affecting the operation of aquaculture facilities. The causes of the power failure, and responses to it, have been used as a case study in risk management for DPI, and prompted other DPI establishments to review risk management for their critical operations.
The project featured outstanding DPI cross-platform collaboration between Marine and Freshwater Sciences and Animal Genetics and Genomics that will continue in new collaborative projects in 2007/08 and beyond. Project staff have become skilled in new techniques. For example, the fish biologists have helped in the checking of genetic data and are now more familiar with applying genetics in fisheries and aquaculture, and the geneticists have helped in the collection of samples and data on fish traits and developed a better understanding of fish husbandry and culture. New skills have been developed in the areas of molecular techniques for sex determination, population biology and data analysis.
4. Conclusions and Recommendations
Murray cod aquaculture is a new and developing industry that exhibits efficient and profitable use of natural resources (water and fish) in regional Victoria. The project has established an excellent foundation for establishing a selective breeding program in Murray cod (family lines and tools for selection). While no selective breeding took place in the current project due to the limited timeframe, the results from this work are the first stage of such a program for the species. Now that markers are linked to traits of interest, a "proof-of-concept" project will need to be undertaken to evaluate the performance of selectively bred strains of Murray cod. With the input of a small amount of research dollars it is anticipated that industry will want to take up this new technology. It is recommended that this research be continued and Industry partners sought to implement the research.
Substantial capability has been developed for this project that can be utilised for other aquaculture programs (eg mussels) and for the study of impacts of the environment and fishing on naturally occurring populations both for freshwater and marine species. It is recommended that this capability be maintained whilst other agencies (eg The Victorian Fisheries Authority) commit to funding other research and development programs.
Results from this study, will assist management of Murray cod populations in the wild for both conservation and recreational fishing purposes. Indeed, a model has already been developed to assess the long-term genetic impacts of various breeding and stocking programs on receiving populations. This model will be used to develop genetically sound stock enhancement programs for Murray cod and other native species for recreational and conservation purposes. Target end-users for this information are primarily fisheries/NRM managers and associated policy makers
5. Appendices
5.1 Technical Reports
5.1.1 Publications
Anon. (2005). High Value Aquaculture in Sustainable Rural Landscapes. Department of Primary Industries, Our Rural Landscape, Tech report. 2 pp.
Bravington, W.J.O. (2007). Evaluation of triploidy induction in brown trout. An internal report prepared for Fisheries Victoria. PIRVic, Snobs Creek.
Bravington, W.J.O., Ingram, B.A., Daly, J., and Mair, G.C. (2006). Application of reproduction technologies to enhance aquaculture of Murray cod. In: Skretting Australasian Aquaculture. Innovation in Aquaculture (Adelaide, 27-30 August 2006). (Poster)
Brown, M. (2005). A Review of Aquacultural Traits in Farmed Murray Cod. University of Melbourne, Industry Project 2005.
Crook, D., Munro, M., Gillanders, B., Sanger, A., Thurstan, S., Macdonald, J., and Ingram, B. (2006). Methods for discriminating between hatchery and wild fish. In: National Workshop on Research and Development Priorities for Stock Enhancement, Fish Stocking and Stock Recovery (Brisbane, 6-7 February 2006), Brisbane.
De Silva, S.S., Nguyen, T.T.T., and Ingram, B.A. (In press). Fish reproduction in relation to aquaculture. In: M. Rocha, A. Arukwe, and Kapoor (Eds), Fish Reproduction. Pp 535-575.
Gooley, G.J., Ingram, B.A., Robinson, N., Hayes, B. and Walker, T. (2006). Seafood biotech futures: R&D led agribusiness opportunities for Victoria? ABIC 2006 Agricultural Biotechnology International Conference 'Unlocking the potential of agricultural biotechnology' (6–9 August 2006, Melbourne).
Ingram, B.A. (2004). Murray cod health in intensive aquaculture systems. In: Management of Fish Health and Diseases in Recirculating Aquaculture Systems, Deakin University, Warrnambool Campus (5-6 June 2004).
Ingram, B.A. (2006). International Travel Report: Genetics in Aquaculture IX. International Symposium on Genetics in Aquaculture (Montpellier, 25-29 Jun 2006). Department of Primary Industries, Internal Report. 18 pp.
Ingram, B.A. (2007). Genetic and reproduction technologies for enhanced production of Murray cod. Our Rural Landscape. Sustainable development through innovation. Technical Note 17. Department of Primary Industries, Melbourne. 4 pp.
Ingram, B.A., Lade, J., Rourke, M., and Robinson, N. (2004). Genetic enhancement of Australian finfish for aquaculture and conservation. In: Australasian Aquaculture: Profiting from Sustainability (Sydney, 26-29 September 2004). P. 167. (Abstract)
Ingram, B.A., McPartlan, H., Rourke, M., Bravington, W., Robinson, N., and Hayes, B. (2006). Genetic enhancement of Murray cod for aquaculture and conservation. In: International Symposium Genetics in Aquaculture IX (26-30 June 2006, Montpellier, France). International Association for Genetics in Aquaculture. P. 45. (Abstract and poster)
Ingram, B.A., McPartlan, H., Rourke, M., Bravington, W., Robinson, N., Nheu, J., H.K., H., and Hayes, B. (2006). Use of genetic and reproduction technologies to conserve and exploit valuable fish resources: a case study featuring Murray cod. In: Skretting Australasian Aquaculture. Innovation in Aquaculture (Adelaide, 27-30 August 2006). (Abstract).
Ingram, B.A., Rourke, M.L., Lade, J., Taylor, A.C., and Boyd, P. (2005). Application of genetic and reproduction technologies to Murray cod for aquaculture and conservation. In: M. Lintermans and B. Phillips (Eds), Management of Murray cod in the Murray-Darling Basin. Statement, recommendations and supporting papers (Workshop held in Canberra, 3-4 June 2004). Murray-Darling Basin Commission, Canberra. Pp 107-109.
Ingram, B.A., Rowland, S.J., Lade, J., and Rourke, M.L. (2005). Management of Australian freshwater cod Maccullochella spp. broodstock for aquaculture and conservation programs. In: World Aquaculture 2005 (9-13 May 2005, Bali, Indonesia). P. 271. (Abstract)
Newman, D., Jones, P., and Ingram, B. (2006). Effectiveness of ultrasonography for identifying sex and assessing maturational status of Murray cod Maccullochella peelii peelii. In: Skretting Australasian Aquaculture. Innovation in Aquaculture (Adelaide, 27-30 August 2006). (Abstract)
Newman, D., Jones, P., Ingram, B., and Smith, B. (2007). Effectiveness of phase-shifted photoperiod regimes for advancing the reproductive development of Murray cod Maccullochella peelii peelii and the independent contributive effects of constant and cycling temperature. In: WAS Aquaculture 2007. Science for Sustainable Aquaculture, San Antonio, Texas, USA (26 Feb. - 2 Mar. 2007). (Abstract)
Nguyen, T.T.T., Baranski, M., Rourke, M., and McPartlan, H. (2007). Characterization of microsatellite DNA markers for a mahseer species, Tor tambroides (Cyprinidae) and cross-amplification in four congeners. Molecular Ecology Notes 7: 109-112.
Rourke, M. and McPartlan, H. (2007). Genetic technologies for the management of natural Murray cod populations. Our Rural Landscape. Sustainable development through innovation. Technical Note 20. Department of Primary Industries, Melbourne. 4 pp.
Rourke, M., Nheu, J., Mountford, H., Lade, L., Ingram, B. & McPartlan, H. (2007) Isolation and characterisation of 102 new microsatellite loci in Murray cod Maccullochella peelii peelii (Percichthyidae), and assessment of cross-amplification in thirteen Australian native and six introduced freshwater species. Molecular Ecology Notes, in press.
Rourke, M., Robinson, N., Taylor, A., Lade, J., and Ingram, B.A. (2004). Use of microsatellite tests to evaluate genetic diversity in wild and captive Murray cod Maccullochella peelii peelii. In: Australasian Aquaculture: Profiting from Sustainability (Sydney, 26-29 September 2004). P. 256. (Abstract)
Rourke, M., Taylor, A., McPartlan, H., and Ingram, B. (2006). Genetic diversity in Murray cod (Maccullochella peelii peelii) across the Murray-Darling Basin. In: Australian Society for Fish Biology Annual Conference (Hobart, Tasmania, 28 August - 1 September 2006). (Abstract)
Rourke, M.L., Lade, J., Ingram, B.A., McPartlan, H., and Taylor, A.C. (2005). Conservation and management of genetic diversity in Murray Cod (Maccullochella peelii peelii). In: International Society for Molecular Biology and Evolution Conference (19-23 June 2005, Auckland, NZ). (Abstract).
Rourke, M.L., Taylor, A.C., McPartlan, H. and Ingram, B.A. (2007). Genetic diversity in Murray cod (Maccullochella peelii peelii) across the Murray-Darling Basin. In: Genetics Society of Australasia Annual Conference (26-29 June, 2007, Sydney) (Abstract).
5.1.2 Publications in Preparation
Bravington, W.J.O., Daly, J.P., Sanchez-Partida, L.G., Gunn, I.M. & Ingram, B.A. (In prep.). Cryopreservation of sperm from Murray cod, Maccullochella peelii peelii (Mitchell) (Percichthyidae): preliminary observations.
Ingram, B.A., Gason, A. & Gooley, G.J. (In prep.) Spawning in captive Murray cod, Maccullochella peelii peelii (Mitchell) (Percichthyidae): seasonality and cues.
Ingram, B.A., Hayes, B. & Rourke, M. (In Prep.) Impacts of stocking enhancement strategies on genetic diversity of Murray cod, Maccullochella peelii peelii and golden perch, Macquaria ambigua.
Ingram, B.A., McPartlan, H., Ho, H.K., Mountford, H. and Hayes, B. (In prep.) Culture performance of communally reared families of Murray cod (Maccullochella peelii peelii).
McPartlan, H., Mountford, H., Nheu, J., Robinson, N., Hayes, B. Ho, H.K. and Ingram,
B.A. (In prep.) Quantitative trait loci for commercially important traits in the Murray cod, Maccullochella peelii peelii (Percichthyidae).
Mountford, H., Ingram, B.A., Nheu, J., Ho, H., Robinson, N., Hayes, B. and McPartlan, H. (In Prep.) A linkage map of Murray cod (Maccullochella peelii peelii) from microsatellite markers.
Newman, D.M., Jones, P.L. & Ingram, B.A. (In prep) Temporal dynamics of oocyte development, plasma sex steroids and somatic energy reserves during seasonal ovarian maturation in captive Murray cod Maccullochella peelii peelii.
Rourke, M., McPartlan, H., Ingram, B.A. & Taylor, A.C. (In prep.) Incidences of polygyny and effective population size in a captive Murray cod population identified using microsatellite DNA markers: implications for wild restocking programs.
Rourke, M, McPartlan, H, Ingram, B. A & Taylor, A. C (In prep) Biogeography and life history ameliorate the potentially negative effects of stocking on a native Australian freshwater fish.
Rourke, M, McPartlan, H, Ingram, B. A & Taylor, A. C (In prep) Population genetic structure of Murray cod (Maccullochella peelii peelii) in the Murray-Darling Basin and evidence of mixed impact of stocking.
5.2 Communication and Evaluation
Team members gave oral presentations on the project at the following events.
5.2.1 International conferences.
International Society for Molecular Biology and Evolution Conference (19-23 June 2005, Auckland, NZ) (Oral presentation).
World Aquaculture 2005 (9-13 May 2005, Bali, Indonesia) (Oral presentation).
International Symposium Genetics in Aquaculture IX (26-30 June 2006, Montpellier, France) (Poster).
ABIC 2006 Agricultural Biotechnology International Conference 'Unlocking the potential of agricultural biotechnology' (6–9 August 2006, Melbourne). (presentation)
5.2.2 National conferences
Management of Murray cod in the Murray-Darling Basin (3-4 June 2004, Canberra) (Poster)
Management of Fish Health and Diseases in Recirculating Aquaculture Systems (5-6 June 2004, Warrnambool) (Oral presentation)
Australasian Aquaculture: Profiting from Sustainability (26-29 September 2004, Sydney) (Oral presentation and poster)
National Workshop on Research and Development Priorities for Stock Enhancement, Fish Stocking and Stock Recovery (6-7 February 2006, Brisbane) (Oral presentation)
Skretting Australasian Aquaculture. Innovation in Aquaculture (27-30 August 2006, Adelaide) (Oral presentation and poster).
Australian Society for Fish Biology Annual Conference (28 Aug - 1 Sep 2006, Hobart) (Oral presentation).
Genetics Society of Australasia Annual Conference (26-29 June, 2007, Sydney) (Oral presentation).
Aquaculture Association of Queensland Annual conference (Harvey Bay, 25-27 August 2007) (invited keynote speaker)
5.2.3 State workshops
ORL 2.3 Future Aquaculture. ORL Forum (28-29 April 2005. Attwood). Risk management in RAS presented to CAS management training workshop (14th June 2005, Attwood) (invited Speaker). Selective breeding in Murray cod. RASnet workshop. (30 April 2005, Snobs Creek).
(recirculating aquaculture systems industry workshop) Murray cod Genetics Workshop. (26 July 2006, Queenscliff) (stakeholder workshop). Murray cod Aquaculture Workshop. (27 April 2006 2006, Knoxfield) (stakeholder
workshop). Our Rural Landscape - Seminars & visit Sunraysia aquaculture sites (Mildura 13 December 2006) (invited speaker) (Murray cod industry workshop)
5.2.4 Media
DPI The Grass. Newsletter of Regional Services and Agriculture. Issue 14 Summer 2004. "Superfish" Page 2.
Primary Voice (A newsletter for those in the Northeast & Goulburn Broken) Winter 2006, P8.
Media release from the Minister for Agriculture, BACK TO THE FUTURE FOR THE MURRAY COD (Friday, 10 February 2006), including regional television and ABC radio interviews. Media released published by several state newspapers and magazines.
ABIC 2006 (Agricultural Biotechnology International Conference). Promotional material for conference 6-9 August 2006, Melbourne.
Media release from the Minister for Innovation and the Minister for Agriculture, SECRET SEX LIFE OF THE MURRAY COD EXPOSED (Tuesday, 8 August 2006), including regional ABC radio interview. Media release published by state, national and international (Asia & Europe) fishing, aquaculture and biotechnology magazines and webpages.
Achievement reports in Annual PIRVic reports: Investigating genetic diversity for conservation management purposes (PIRVic - Annual Review 2003/04), Genetic strategies select Murray cod for aquaculture (PIRVic Achievement Report 2004/05), Genetic Strategies Identify Murray cod and Australian bass or estuary perch? (PIRVic Achievement report 2005-06), Only DNA has the answer! (PIRVic Achievement report 2005-06) and Fish tale with major export potential (DPI Agriculture and Food Sector Investment Report 2005-06).
5.3 References
Bearlin, A.R. & Tikel, D. (2003) Conservation genetics of Murray-Darling basin fish; silver perch (Bidyanus bidyanus), Murray cod (Maccullochella peelii) and trout cod
(M. macquariensis) In Managing Fish Translocation and Stocking in the Murray-Darling Basin. Workshop held in Canberra 25-26 September 2002. Statement, Recommendations and Supporting Papers (Phillips, B.F. ed.), pp. 59-83. WWF Australia, Sydney.
Brown, M. (2005) A Review of Aquacultural Traits in Farmed Murray Cod. University of Melbourne, Industry Project 2005.
Douglas, J.W., Gooley, G.J., Ingram, B.A., Murray, N. & Brown, L.D. (1995) Natural hybridisation between Murray cod, Maccullochella peelii peelii (Mitchell), and trout cod Maccullochella macquariensis (Cuvier), (Percichthyidae), in the Murray River, Australia. Marine and Freshwater Research, 46, 729-734.
Goudet, J. (1995) Fstat version 1.2: a computer program to calculate Fstatistics. Journal of Heredity, 86, 485-486.
Ingram, B.A. & De Silva, S.S. eds. (2004) Development of Intensive Commercial Aquaculture Production Technology for Murray cod. Final Report to the Fisheries Research and Development Corporation (Project No. 1999/328). Primary Industries Research Victoria, DPI, Alexandra, Victoria, Australia.
Ingram, B.A. & Rimmer, M.A. (1992) Induced breeding and larval rearing of the endangered Australian freshwater fish trout cod, Maccullochella macquariensis (Cuvier) (Percichthyidae). Aquaculture and Fisheries Management, 24, 7-17.
Kligerman, A.D. & Bloom, S.E. (1977) Rapid chromosome preparations from solid tissues of fishes. J. Fish. Res. Board Can. , 34, 266-269.
Loughnan, S.R., Baranski, M.D., Robinson, N.A., Jones, P.L. & Burridge, C.P. (2004) Microsatellite loci for studies of wild and hatchery Australian Murray cod Maccullochella peelii peelii (Percichthyidae). Molecular Ecology Notes.
Marshall, T.C., Slate, J., Kruuk, L. & Pemberton, J.M. (1998) Statistical confidence for likelihood-based paternity inference in natural populations. Molecular Ecology, 7, 639-655.
Raymond, M. & Rousset, F. (1995) GENEPOP (version 1.2): population genetic software for exact test ecumenicism. Journal of Heredity, 86, 248-249.
Robb, D.H.F. (2001) Measurement of fish flesh colour In Farmed Fish Quality (Kestin,
S.C. & Warriss, P.D. eds.), pp. 298-306. Fishing News Books, Oxford.
Rourke, M., Nheu, J., Mountford, H., Lade, L., Ingram, B. & McPartlan, H. (2007) Isolation and characterisation of 102 new microsatellite loci in Murray cod Maccullochella peelii peelii (Percichthyidae), and assessment of cross-amplification in thirteen Australian native and six introduced freshwater species. Molecular Ecology Notes, in press.
Rowland, S.J. (1984) The hormone-induced spawning of silver perch, Bidyanus bidyanus (Mitchell) (Teraponidae). Aquaculture, 42, 83-86.
Rowland, S.J. (1986) The hormone-induced spawning and larval rearing of Australian freshwater fish, with particular emphasis on golden perch, Macquaria ambigua In Proceedings of the First Freshwater Aquaculture Workshop (Narrandera, NSW, 2125 February 1983) (Reynolds, L.F. ed.), pp. 23-32. Department of Agriculture NSW, Sydney.
Schneider, S., Roessli, D. & Excoffier, L. (2000) Arlequin ver 2.000: A software for population genetics data analysis, Genetics and Biometry Department, University of Geneva, Switzerland.
5.4 Financial Reports
| Fund source | Budget 03/04 | Exp yr 1 | Budget yr 2 | Exp yr 2 | Budget yr 3 | Exp yr 3 | Budget yr 4 | Exp yr 4 |
|---|---|---|---|---|---|---|---|---|
| 31 | 76,164 | 85,258 | 70,000 | 72,325 | 76,463 | 63,172 | 75,000 | 75,000 |
| 34 | 369,565 | 437,049 | 519,591 | 503,020 | 513,384 | 513,384 | 530,000 | 532,201 |
| other | 5,000 | 5,000 | 5,000 | 5,000 | ||||
| Total | 445,729 | 522,307 | 594,591 | 580,345 | 594,847 | 581,556 | 605,000 | 607,201 |
Project 05194: High value aquaculture in sustainable rural landscapes
Project Title: High value aquaculture in sustainable rural landscapes
Project MIS No: 05194
Author: Brett A. Ingram,
Helen McPartlan,
Hui King Ho,
Meaghan Rourke, and
William Bravington
ISBN 978-1-74199-509-1 (Print)
ISBN 978-1-74199-510-7 (Online)
Acknowledgments: The authors are pleased to acknowledge the financial and inkind support from The Victorian Fisheries Authority (cofunder), Holsworth Wildlife Research and NSW Recreational Fishing Trust Fund, DSE, DPI-NSW, DPI-Qld, PIRSA, recreational angling clubs, Monash University, Melbourne University, Flinders University, Monash Institute of Medical Research, Deakin University, RMIT, La Trobe University, Cryologic Ltd, representatives of the aquaculture Industry in Victoria, and students and staff.
