Identifying the spawning locations of King George whiting in Victorian waters

NO. 21
JULY 2004
Paul A Hamer, Gregory P Jenkins, KP Sivakumaran
ISSN 1329-7287
ISBN 1 74146 298 3
Preferred way to cite this publication:
Hamer PA, Jenkins GP, Sivakumaran KP (2004) Identifying the spawning locations of a recreational fishing based study. Fisheries Victoria Assessment Report Series No. 21.
Executive Summary
King George whiting, Sillaginodes punctata, are one of the most important recreational and commercial finfish species of bays and inlets in southeastern Australia. While over the last decade our understanding of the life cycle of this species has increased dramatically, there remains one large gap, and that is, "where do they spawn?" Our current understanding of the life cycle is that the juveniles, up to about 3 years of age, inhabit sheltered waters of bays and inlets, and adults, greater than 3 years, inhabit open coastal waters. In Victoria, larval stages consistently enter bay and inlet nursery areas from ocean waters during the spring at around 20 mm in length and 100 -170 days of age. Based on modelling of larval drift it is predicted that most of the juveniles that constitute the Victorian fishery are derived from spawning grounds in the far west of Victoria and South Australia. The only known spawning areas for King George whiting in southeastern Australia occur in South Australian waters. This has lead to the question, "does the Victorian fishery depend primarily on the spawning stock in South Australia?" Unlike Victoria, large adults are heavily exploited in South Australian waters. If there are no major spawning areas in Victorian waters it could mean that the sustainability of the Victorian King George whiting fishery depends on the careful management of adult spawning stocks in South Australian waters.
The aim of this project was to investigate the extent and timing of spawning by King George whiting in Victorian waters. Because there is no significant commercial fishery for adult King George whiting in Victorian coastal waters, the major source of knowledge on the distribution of adult fish in coastal waters lies with the recreational anglers who target the species. We enlisted the help of some 60 recreational anglers who fish in Victorian coastal waters and the entrance regions to major bays and inlets. These anglers were supplied with equipment and instructed in the collection of fish frames for the examination of reproductive condition.
During the period November 2002 – June 2004 we obtained over 1,600 samples from anglers across a broad geographic area spanning approximately 800 km of the Victorian coast. Most samples were obtained during the expected spawning period of March – June.
The size and age of samples ranged from 25 – 57 cm fork length, and 2 to 11 years respectively. All but two individuals were between 3 and 6 years age. Only one female was found to be running ripe (about to spawn), and no fully mature males were found. The one ripe female was atypical in size (57 cm fork length, 1.85 kg) and age (11 years). Several other females had developed gonads during the expected spawning season, but there was no evidence that these fish were close to spawning. Histological examination of ovaries suggested that most fish were either immature or in the early stages of development. Some developed females contained yolked oocytes (eggs) in atresia but no post-ovulatory follicles, suggesting that they had developed but failed to spawn. This has been observed in South Australian populations and is suggested to occur when the cues required for spawning are not present. These cues are suggested to be; the presence of older reproductive fish and ripe males, and habitat that is characterised by an exposed nature, deep-water and reef.
While most of our samples came from anglers fishing in water less than 20 m depth we also sampled fish from deeper water (30 – 40 m) at two locations. The results for these fish were similar to the inshore samples. The absence of older fish (6 – 17 years age) may be important to explaining the lack of spawning observed in Victoria. Age data showed that only 4 year classes (3 – 6 years) dominated the coastal fishery, and the age of fish increased in a westerly direction. These observations are consistent with a gradual migration to the west.
The question of how dependent the Victorian fishery is on spawning stock in South Australian waters still remains unclear. Although it is possible that spawning does occur at some locations in Victorian waters, our results are consistent with the idea that spawning is predominantly occurring South Australia. While it would be difficult to identify highly localised spawning areas, particularly in deeper waters, there are other ways to clarify the reason why spawning fish were not found in significant numbers during this study. The simplest approaches would involve tag/recapture or otolith chemistry to determine if Victorian fish are migrating back to South Australian spawning grounds.
Table of Contents
Executive Summary
Introduction
Project Design and MethodsInvolvement of recreational anglers - publicity
Sampling design
Collection of fish
Reproductive condition of fish
Processing of fish
Reproductive staging
Reproductive cycle
Fecundity estimation
Age and Growth
Results
Fish collections
Gonad development
Age and length
Age
Length
Age / length relationship
Discussion
Implications for management
Conclusions
Acknowledgements
References
Appendix 1 – Details of publicity and extension related to the projectAppendix 2 – Keys used for reproductive staging of King George whiting
Introduction
King George whiting (Sillaginodes punctata) occur along the southern coast of mainland Australia (Kailola et al. 1993). It is commonly regarded as the most valuable inshore finfish species of southern Australia (Fowler et al. 1999), and is taken in substantial quantities by recreational fishers (Jones et al. 1990, McGlennon and Kinloch 1997). Most of the King George whiting caught in Victorian bays and inlets are less than 40 cm in length and 3 years of age. The recreational fishery for this species in Victoria is, therefore, primarily based on subadult fish. Large, adult fish in spawning condition are rarely caught because it is thought that these reproductively active fish may move offshore (although no study has examined this).
The spawning areas and times estimated for King George whiting caught in Port Phillip Bay, Western Port and Corner Inlet (Fig. 1) have previously only been identified using indirect methods. Research by Jenkins and May (1994) has shown that the daily rings on ear-bones (otoliths) of juvenile King George whiting in Port Phillip Bay provide an estimate of the length of time of the larval phase in Bass Strait prior to entering a bay, and thereby an estimate of the time of spawning. KGW larvae entering Port Phillip Bay were aged between 100 and 170 days and were predicted to have been spawned between April and July (Jenkins and May 1994). Computer modelling of the currents in Bass Strait predicted that juvenile King George whiting in Port Phillip Bay, Western Port and Corner Inlet could have been spawned in western Victoria and southeastern South Australia (Jenkins et al. 2000). The centre of the predicted spawning distribution was approximately 500 km west of the bays, with a region of intense spawning spread along approximately 300 km of coastline (Jenkins et al. 2000). Interestingly, however, the model also predicted that juveniles in Corner Inlet could have been spawned close by (Wilsons Promontory, Fig. 1). To date, however, the spawning times and locations of King George whiting in Victoria have not been measured directly, leaving a major gap in our understanding of the biology of this species.
Research is required that directly measures the reproductive state of adult King George whiting along the Victorian coast so that the spawning times and locations of King George whiting in Victorian waters can be determined. A direct method of investigating spawning times and areas is by studying gonad condition. It is possible to assess the stage of gonad development and the imminence of spawning in fish using macro- and microscopic characteristics of gonads from male and female fish (Hunter and Macewicz 1985). This type of research has been conducted for many of our commercially important species (Farely and Davis 1998). Major studies of this type have been carried out for King George whiting in Western Australia (Hyndes et al. 1998) and South Australia (Cockrum and Jones 1992, Fowler et al. 1999, 2000a). In this study we examine the reproductive characteristics of adult fish collected by recreational anglers along the Victorian coastline. The aim of this work is to identify whether King George whiting spawn along the coastline of Victoria, and if so, where and when does spawning take place.
This research has important implications for the sustainable management of this fishery in Victoria's bays and inlets. In particular, management of the Victorian fishery requires a greater understanding of the link between the Victorian fishery and the only known spawning areas which are situated in South Australian waters (Fowler et al. 1999). Adult King George whiting are not heavily exploited in Victorian coastal waters, whereas they are in South Australian waters. If spawning is not occurring in Victorian waters it would suggest that the Victorian fishery is dependent on larvae that are transported by ocean currents from spawning areas in South Australian waters (Fowler et al. 1999). Under this scenario the sustainability of the Victorian fishery could depend on careful management of spawning adults in South Australian waters.
Project Design and Methods
Involvement of recreational anglers - publicity
To encourage participation of recreational anglers we publicised this project through a range of media, conducted presentations at angling clubs and attended several major fishing competitions (Appendix 1). Through the initial publicity we attracted some 60 anglers who expressed interest in becoming involved in collection of samples. These anglers were added to a database and all were sent information/collection kits. Collection kits included a brief background to the project, laminated pictures of reproductive King George whiting (i.e. Fig. 2), a flyer to show other anglers how to get involved, specially designed plastic bags for storage of frames, labels and pencils for recording capture locations and dates, and rubber bands for sealing sample bags. We also targeted several diary anglers already involved in research activities at PIRVic - MaFS. Towards the period when spawning was expected to begin we encouraged greater involvement of several coastal angling clubs (Appendix 1) by inviting them to conduct King George whiting focussed competitions with prizes of fishing tackle donated from the project funds. Updates on progress of the project were regularly provided to anglers involved in collection via phone conversations and face to face contact during the collection of samples.
Sampling design
In order to identify the times of spawning of King George whiting, large fish (>30 cm) were sampled from volunteer recreational anglers from November to July. This period encompassed the April-July spawning period identified in South Australian King George whiting (Fowler et al. 1999, 2000ab), and predicted for Victoria based on otolith ageing of small larvae (Jenkins and May 1994, Jenkins et al. 2000). Based on earlier research, spawning was expected to occur in ocean waters or towards the entrances of large bays and gulfs (Jenkins et al. 1998, Fowler et al. 2000ab), therefore, collections were concentrated in coastal waters and the entrance regions of bays such as Port Phillip and Western Port.
Previous modelling suggested that King George whiting larvae that recruit to bays and inlets in central Victoria are spawned from south-east of SA to central Victoria (Jenkins et al. 2000). Even though large whiting are caught along the Victorian coast, it is not known whether these fish actually spawn in Victorian waters, and therefore whether they contribute directly to the fishery in this region. To address where KGW spawn in Victorian waters, we sampled fish from a broad geographic range of locations along the Victorian coast. These included Portland, Port Fairy, Warrnambool, Apollo Bay, Lorne, Torquay, southern Port Phillip Bay and Western Port, Waratah Bay, Corner Inlet, Shallow Inlet and the eastern side of Wilson's Promontory (Fig. 1). Details of collections are included in Table 1.
Collection of fish
Adult King George whiting carcasses were collected from recreational fisherman from November to July 2002-2003. In 2004 frames were collected from a reduced number of locations and times following the results of 2002-2003 (Table 1). In May/June 2004 we also collected specimens from commercial catches from deeper coastal waters at two locations, East Wilsons Promontory and Apollo Bay (Fig. 1). Anglers were asked to keep their entire catch where possible or a random sample of as many fish as they could store. We also requested that they retain any exceptionally large fish for ageing purposes. Anglers were instructed to weigh fish where possible prior to filleting and to freeze the filleted frames with internal organs intact in the labelled (location, date, angler) storage bags provided. Frames were collected on a monthly basis, and anglers were requested to call us immediately if they captured a fish with obvious signs of gonad development.
Reproductive condition of fish
PROCESSING OF FISH
Laboratory processing involved measurement of frames for fork (FL) and total length (TL), and weighing of whole fish. Otoliths (earbones) were removed for ageing and gonads were removed from selected specimens and preserved in formalin for histological examination. Gonads from all samples were sexed and weighed to the nearest 0.1 g.
REPRODUCTIVE STAGING
Reproductive condition was assessed by macroand microscopic examination. Macroscopic examination followed a 5 stage key (Appendix 2a) developed previously by Fowler et al. (1999) for King George whiting. Macroscopic examinations involved classification based on colouration and size of gonads, and the visibility and appearance of oocytes (eggs) in females (Appendix 2a). For microscopic examination the gonads were preserved in 10% neutral buffered formalin and stored for at least 2 weeks. The samples were sent to a commercial pathology service for mounting and sectioning and then returned for subsequent histological examination. Briefly, a section of the transverse medial material was blocked in paraffin wax and 6 ìm sections were cut, mounted and stained in Harris' haematoxylin and eosin (Luna, 1968). This enabled examination of the processes occurring at cellular level during the different stages of the reproductive cycle. Microscopic analyses followed the key presented in Appendix 2b, which is modified from Knuckey and Sivakumaran (2001) and follows descriptions presented in Macewicz and Hunter (1985), and Fowler et al. (1999).
REPRODUCTIVE CYCLE
Gonosomatic indices (GSI's) were calculated following the equation; GSI = (Wg/Wf)*100, where Wg is gonad weight and Wf is total fish weight (Fowler et al. 1999). GSI was plotted through time based on data pooled across sampling areas. Because we received many filleted frames without information on weight, we converted lengths to weights based on a regression of FL versus Wf for samples of 100 male and 110 female fish. The data required log10 transformation to produce linearity. FL and W were highly correlated for both sexes (r2 = 0.96/0.93, males/females). The relationships between FL and Wf of King George whiting from 29 to 46 cm FL are described by the equations:
Males – log10(Wf)= log10 (FL)*3.486 – 6.357
Females – log10(Wf)= log10 (FL)*3.401 – 6.357
FECUNDITY ESTIMATION
The total fecundity was estimated for one gravid female sampled in late February 2003 (FL 57 cm). Total fecundity was estimated by multiplication of the number of yolked, nuclear migrated and hydrated oocytes in a 2g sample of gonad tissue taken across 10 regions of the gonad.
Age and Growth
Annual increment formation has previously been validated for King George whiting by Fowler and Short (1998). We aged individual King George whiting by counting annual increments in sectioned otoliths (sagittae). Birth date was fixed at 1st July (i.e. end of spawning season). We aged 317 individuals that were made up of 4 samples of 60-100 randomly selected individuals from each two-month period from November 02 to June 03. Age and length were compared among sampling locations to examine trends in age and length with distance from the major juvenile nursery areas in central Victoria; Port Phillip Bay and Western Port. We also deliberately aged several of the largest fish to determine the maximum age of fish sampled.
The von-Bertalanffy growth curve, which describes the age/length relationship, was constructed from the data obtained during this project combined with previous data from ageing of King George whiting at PIRVic-MaFS. This meant that the growth curve was developed for ages 1 to 11, rather than 3 to 11, therefore producing a more accurate picture of the age/length relationship for Victorian King George whiting. Because fish were sampled at different times the age/length relationship was determined for ages in decimal years based on the date of capture and the birth date of 1st July (i.e. the end of spawning season).
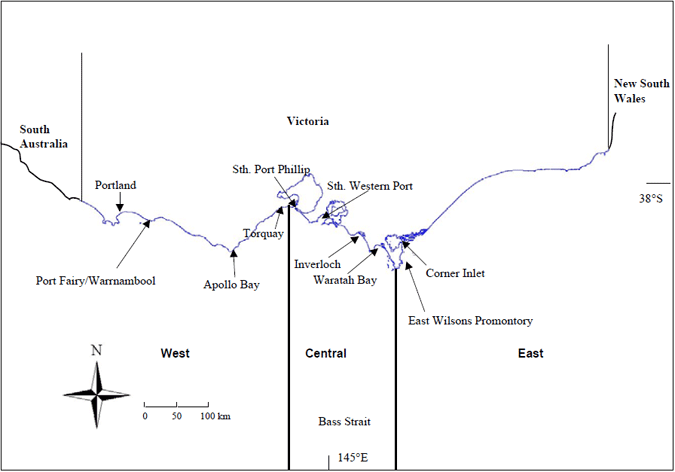
Figure 1. Map of the Victorian coast showing locations and zones where samples of King George whiting were collected from 2002-2004.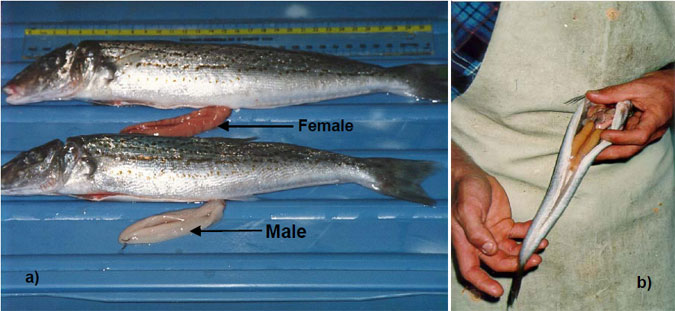
Figure 2. a) Images of reproductively mature King George whiting that were supplied to anglers involved in collection of frames (photo courtesy of Dr. Tony Fowler, South Australian Research and Development Institute), b) developing female collected during April in Victorian coastal waters.
Results
Fish collections
Approximately 1,600 King George whiting were collected from fishermen between November 2002 and June 2004 (Table 1). The majority of the frames were supplied by anglers fishing in the western and central zones (Fig.1, Table 1). Over 970 frames were collected during the expected spawning season of March-June (Table. 1). Most recreational samples came from depths shallower than 20 m. However, we obtained 110 whole fish samples from commercial fishermen that were captured in depths from 35-40 m. These deep water samples came from two locations, the East Wilson's Promontory and directly off Apollo Bay, and were collected in May/June (Fig.1, Table 1). The overall sex ratio of samples was 47% males and 53% females.
Gonad development
The results of macroscopic and microscopic examinations provided little evidence for spawning activity in inshore waters along the Victorian coast. Although there were signs of gonad development beyond the immature stage (macroscopic stage 2, 3, Appendix 2a), only one specimen was collected in running ripe condition (macroscopic stage 4, Appendix 2a). This specimen was an atypical, exceptionally large fish (57 cm FL, 1.85 kg) taken near the entrance to Western Port. This fish was approaching 11 years old making it the oldest King George whiting aged from Victorian waters. The ovaries from this specimen were exceptionally large, weighing a total of 398 g, and one ovary was considerably larger than the other, suggesting an abnormality. We estimated the total fecundity of this individual to be 359,052 oocytes (including yolked, nuclear migrated and hydrated oocytes). Unfortunately, due to the state of preservation of this sample when we received it, we could not accurately determine its batch fecundity (i.e. number of hydrated oocytes, or eggs likely to be spawned on the day of capture) or conduct histological examination.
The majority of the females sampled were in the undeveloped (macroscopic stage 1, Appendix 2a, Table 1), or early developing stage (macroscopic stage 2, Appendix 2a, Table 1) from all the areas and all months. The majority of males were undeveloped or immature stage (macroscopic stage 1, Appendix 2, Table 1), although some males of macroscopic stage 2 were found during the expected spawning period of March - June (Table 1).
Examinations of histological sections showed that developed ovaries staged as macroscopic 3 contained only unyolked and yolked oocytes in atresia and no evidence of postovulatory follicles (Fig. 3ab). This suggests that a high level of development was reached but there was failure to spawn. The majority of developing ovaries classified as macroscopic stage 2 contained only unyolked oocytes (perinucleolar stages) (Fig. 4), but a few of the stage 2 ovaries had both unyolked and yolked oocytes in atresia.
Females with developing ovaries (macroscopic stage 3 and above, Appendix 2) were only found in samples collected from late February to June (Table 1). Due to the lack of spawning adults there was low variation in the GSI's across the sampling period for both males and females (Fig. 5a). There was a slight increase in GSI's for both males and females over the expected spawning period of February to June, but the average GSI remained well below that which would indicate spawning activity, i.e. values of 4 to 8 (Fowler et al. 1999) (Fig. 5a). Ovary weights were generally below 20 g, with most below 10 g (Fig. 5b). There was a linear relationship between ovary weight and ovary free fish weight (r = 0.70, p < 0.001) (Fig. 5b). Developing fish were the obvious outliers from this linear relationship, i.e. black squares and circles in Fig. 5b.
Table 1. Details of numbers, sizes, sex and macroscopic reproductive condition of adult King George whiting collected by recreational anglers along the Victorian coast, 2002-2004.
| Zone | Month/ Year | Location | N total | N M | N F | FL cm (mean / SD) | Macroscopic stages (N - M/F) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||||||
| West | Nov 02 | Portland | 49 | 27 | 22 | 40.8 (1.6) | 27/2 | 0/2 | |||
| Nov 02 | Port Fairy | 6 | 3 | 3 | 41.7 (2.5) | 3/3 | |||||
| Nov 02 | Apollo Bay | 37 | 22 | 15 | 40.5 (2.3) | 24/7 | 0/6 | ||||
| Nov 02 | Torquay | 14 | 8 | 6 | 39.7 (4.4) | 8/6 | |||||
| Dec 02 | Port Fairy | 9 | 5 | 4 | 38.4 (3.1) | 5/4 | |||||
| Dec 02 | Torquay | 9 | 3 | 6 | 38.2 (2.9) | 3/6 | |||||
| Jan 03 | Port Fairy | 12 | 8 | 4 | 39.2 (2.8) | 8/1 | 0/3 | ||||
| Feb 03 | Torquay | 10 | 3 | 7 | 38.7 (4.2) | 3/3 | 0/4 | ||||
| Mar 03 | Apollo Bay | 62 | 34 | 28 | 37.5 (2.0) | 28/23 | 6/5 | ||||
| Mar 03 | Lorne | 6 | 5 | 1 | 38.1 (2.1) | 3/0 | 2/1 | ||||
| Mar 03 | Torquay | 10 | 2 | 8 | 39.3 (2.9) | 2/6 | 0/6 | ||||
| Apr 03 | Port Fairy | 11 | 5 | 6 | 38.6 (3.3) | 4/1 | 0/5 | 1/0 | |||
| Apr 03 | Lorne | 73 | 42 | 31 | 38.1 (1.9) | 40/6 | 3/24 | ||||
| Apr 03 | Torquay | 12 | 5 | 7 | 37.0 (1.9) | 5/5 | 0/2 | ||||
| May 03 | Port Fairy | 47 | 17 | 30 | 38.9 (2.6) | 13/2 | 4/28 | ||||
| May 03 | Apollo Bay | 43 | 25 | 18 | 38.8 (2.2) | 20/0 | 5/18 | ||||
| May 03 | Lorne | 12 | 6 | 6 | 39.2 (1.8) | 5/4 | 1/2 | ||||
| May 03 | Torquay | 3 | 0 | 3 | 38.3 (3.8) | 0/3 | 0/0 | 0/1 | |||
| June 03 | Torquay | 4 | 2 | 2 | 38.4 (2.0) | 2/0 | 0/2 | ||||
| July 03 | Torquay | 3 | 0 | 3 | 38.6 (6.4) | 0/0 | 0/3 | ||||
| Dec 03 | Lorne | 8 | 3 | 5 | 39.5 (1.9) | 3/0 | 0/5 | ||||
| Jan 04 | Lorne | 16 | 11 | 4 | 35.3 (3.0) | 11/3 | 0/1 | ||||
| Jan 04 | Portland | 10 | 4 | 6 | 40 (2.8) | 4/4 | 0/2 | ||||
| Feb 04 | Lorne | 12 | 4 | 8 | 35.3 (1.9) | 4/6 | 0/2 | ||||
| Mar 04 | Lorne | 30 | 14 | 16 | 36.6 (2.6) | 14/8 | 0/8 | ||||
| Mar 04 | Apollo Bay | 35 | 22 | 13 | 36.3 (2.1) | 22/0 | 0/12 | 0/1 | |||
| Apr 04 | Lorne | 26 | 20 | 6 | 37.7 (2.1) | 18/6 | 2/0 | ||||
| May 04 | Lorne | 10 | 5 | 5 | 37.8 (1.6) | 5/4 | 1/0 | ||||
| May 04 | Apollo Bay - deep | 11 | 7 | 4 | 35.3 (1.9) | 6/1 | 1/3 | ||||
| June 04 | Apollo Bay - deep | 17 | 6 | 11 | 39.2 (2.2) | 2/0 | 4/11 | ||||
| Central | Nov 02 | Sth West Port | 10 | 4 | 6 | 36.1 (2.7) | 4/6 | ||||
| Nov 02 | Shallow Inlet | 12 | 7 | 5 | 32.6 (4.5) | 7/5 | |||||
| Dec 02 | Sth West Port | 8 | 4 | 4 | 39.2 (3.2) | 4/2 | 0/2 | ||||
| Dec 02 | Inverloch | 20 | 7 | 13 | 33.4 (4.1) | 6/9 | 0/4 | ||||
| Jan 03 | Sth West Port | 35 | 14 | 21 | 35.0 (4.2) | 14/15 | 0/6 | ||||
| Jan 03 | Shallow Inlet | 17 | 5 | 7 | 32.5 (4.3) | 5/7 | |||||
| Feb 03 | Sth West Port | 63 | 29 | 34 | 34.2 (4.2) | 29/25 | 0/8 | 0/1 | |||
| Mar 03 | Sth Port Phillip | 17 | 8 | 9 | 35.2 (2.6) | 8/2 | 0/5 | 0/2 | |||
| Mar 03 | Sth West Port | 55 | 28 | 37 | 33.5 (3.6) | 18/32 | 0/5 | ||||
| Mar 03 | Shallow Inlet | 8 | 5 | 3 | 36.2 (3.1) | 5/3 | |||||
| Apr 03 | Sth West Port | 39 | 20 | 19 | 35.2 (3.0) | 20/16 | 0/3 | ||||
| Apr 03 | Waratah Bay | 17 | 3 | 14 | 38.8 (2.9) | 1/3 | 2/11 | ||||
| May 03 | Sth Port Phillip | 33 | 12 | 21 | 35.0 (4.7) | 11/11 | 1/10 | ||||
| May 03 | Sth West Port | 51 | 26 | 25 | 35.6 (3.7) | 26/17 | 0/8 | ||||
| May 03 | Waratah Bay | 4 | 2 | 2 | 36.5 (3.3) | 2/0 | 0/2 | ||||
| June 03 | Sth Port Phillip | 32 | 16 | 16 | 34.4 (2.8) | 16/12 | 0/4 | ||||
| July 03 | Sth Port Phillip | 31 | 8 | 23 | 33.4 (4.4) | 8/0 | 0/10 | ||||
| Oct 03 | Sth Port Phillip | 14 | 7 | 7 | 36.4 (2.1) | 7/2 | 0/5 | ||||
| Dec 03 | Sth West Port | 62 | 17 | 45 | 37.2 (3.2) | 17/11 | 0/34 | ||||
| Feb 04 | Sth West Port | 7 | 3 | 4 | 38.5 (2.1) | 0/1 | 3/3 | ||||
| Feb 04 | Sth Port Phillip | 20 | 8 | 12 | 36.9 (2.1) | 8/1 | 0/11 | ||||
| May 04 | Sth West Port | 155 | 75 | 80 | 36.5 (3.4) | 73/42 | 2/38 | ||||
| East | Nov 02 | Corner Inlet | 15 | 8 | 7 | 36.2 (1.5) | 8/7 | ||||
| Jan 03 | Corner Inlet | 57 | 28 | 29 | 28.2 (1.3) | 28/29 | |||||
| Apr 03 | Cornet Inlet | 51 | 24 | 27 | 31.8 (1.4) | 24/27 | |||||
| May 03 | Corner Inlet | 39 | 21 | 18 | 32.2 (1.7) | 21/17 | 0/1 | ||||
| June 03 | Wilsons Prom - deep | 30 | 30 | 15 | 33.4 (1.3) | 15/3 | 0/12 | ||||
| May 04 | Corner Inlet | 15 | 6 | 9 | 32.6 | 6/5 | 0/8 | ||||
| May 04 | Wilsons Prom - deep | 52 | 19 | 19 | 35.5 (2.5) | 15/25 | 4/7 | 0/1 | |||
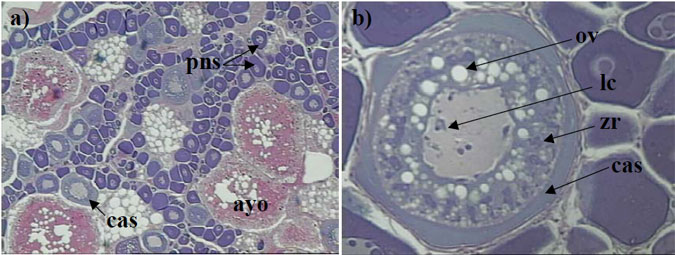
Figure 3. a) Histological section of the ovary from a female King George whiting collected in May that was classified as macroscopic stage 3 (magnification x 100), b) close up of a cortical alveoli or developing oocyte stage (magnification x 250).
Features to note in a) are the perinucleolar (pns) or immature oocytes, the cortical alveoli (cas) or developing oocytes, and the atresia of yolked oocytes (ayo). The absence of post-ovulatory follicles (POF's) and the presence of atresia of yolked oocytes suggest that this fish had developed close to the spawning stage but did not spawn. In b) the cortical alveoli is the pale-blue-stained cytoplasm (cas), the pink-stained material is zona radiata (zr), oil vesicles (ov) are appearing, and lampbrush chromosomes (lc) are visible in the nucleus.
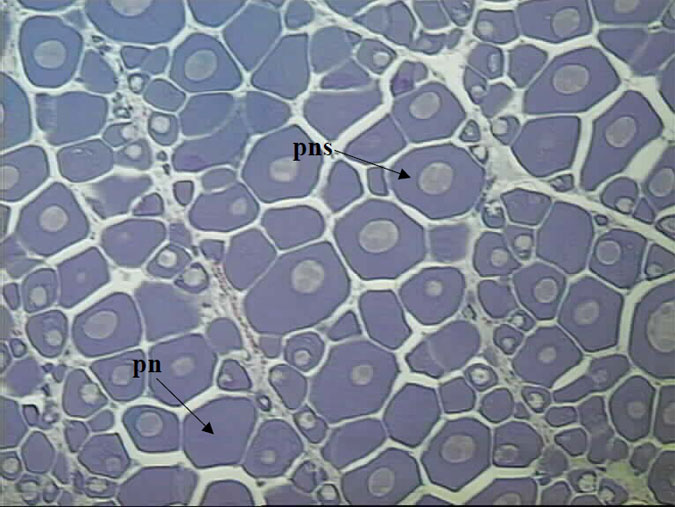
Figure 4. Histological section of a female King George whiting ovary collected in April and classified as macroscopic stage 2 (magnification x 100).
The ovary is dominated by the perinucleolar stage (pn). This stage is immature, although some oocytes have increased in size slightly with obvious nucleoli appearing (pns).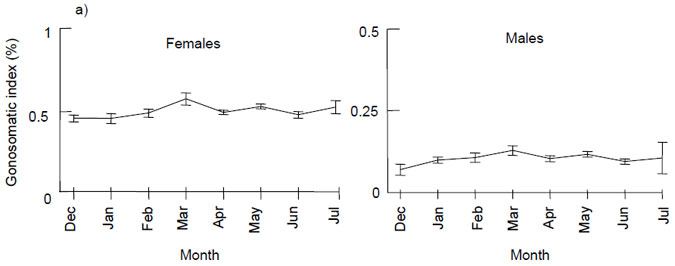
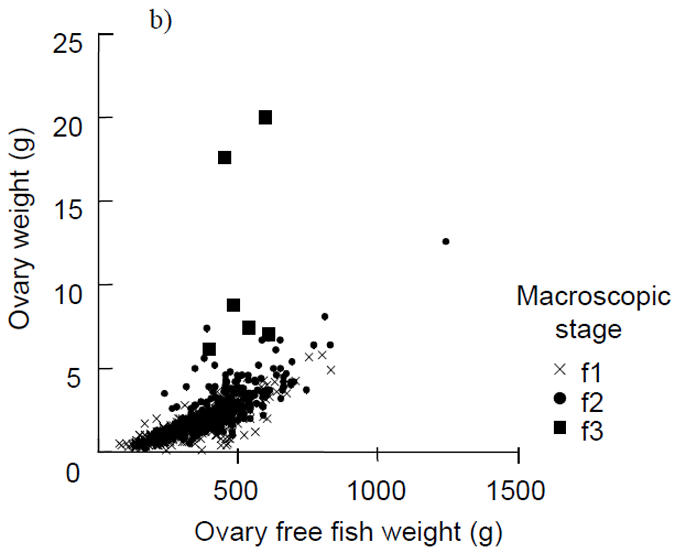
Figure 5. a) Mean (± 1 standard error) gonosomatic indices for female and male King George whiting sampled along the Victorian coast from December to July, b) relationship between ovary weight and ovary free fish weight.
Data are pooled across sampling locations. The one ripe fish captured in late February is not included due to having a much higher GSI (GSI value 25) and ovary weight (398 g) than all other samples.
Age and length
AGE
The majority of fish sampled were between 3 and 6 years of age, and there were very few fish above 6 years age (Fig. 6 ab). The two largest specimens collected (54 and 57 cm FL) were also the oldest (age classes 10 and 11 respectively). Older fish (i.e. age classes 5 and 6) were more common in samples taken in the western zone, than those taken in the central zone (Fig. 6a). Age distributions were similar for males and females (Fig. 6b).
The mean age of fish was lowest for the southern end of Port Phillip Bay and increased with distance to the west of Port Phillip Bay (Fig. 7). The highest mean age was for Portland fish (Fig. 7). Fish from Waratah Bay were predominantly 4 year-old (Fig 7). We did not age any fish from Corner Inlet, but based on their size and previous ageing work we would be confident that the majority of these fish were of age 3 years and younger (see Figs. 8b and 9b).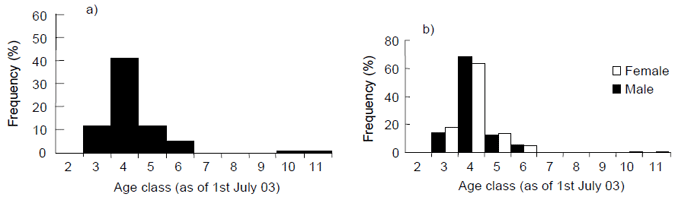
Figure 6. a) Age frequency distribution for King George whiting sampled by recreational anglers from Victorian coastal waters and the entrance regions of Port Phillip and Western Port Bays, November 2002 - June 2003, and b) age frequency by sex, n=317.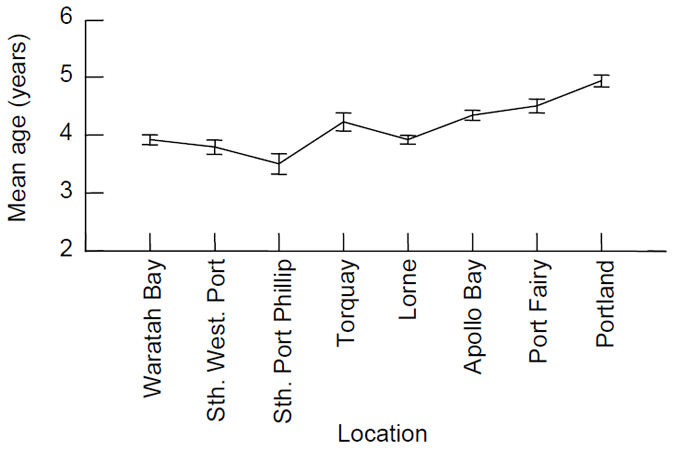
Figure 7. Comparison of the mean age (± 1 standard error) of King George whiting collected by recreational anglers across sampling locations from east to west.
Ages are based on the birth date of 1st July and standardised to 1st July 2003.
LENGTH
The length frequency distributions of males and females collected for examination of reproductive condition were similar, ranging from 25 to 47 cm FL. Only two fish were collected over 50 cm FL (Fig. 8 ab). Size distributions for both sexes had modes between 35 and 40 cm FL (Fig. 8 ab).
The mean lengths of fish samples from locations within bays and inlets, (i.e. Corner Inlet, Shallow Inlet, Inverloch, Sth. Western Port and Sth. Port Phillip) were lower than for samples from the oceanic sites (Fig. 8b). There was a slight trend for increasing mean size to the west of Port Phillip Bay (Fig. 8b)
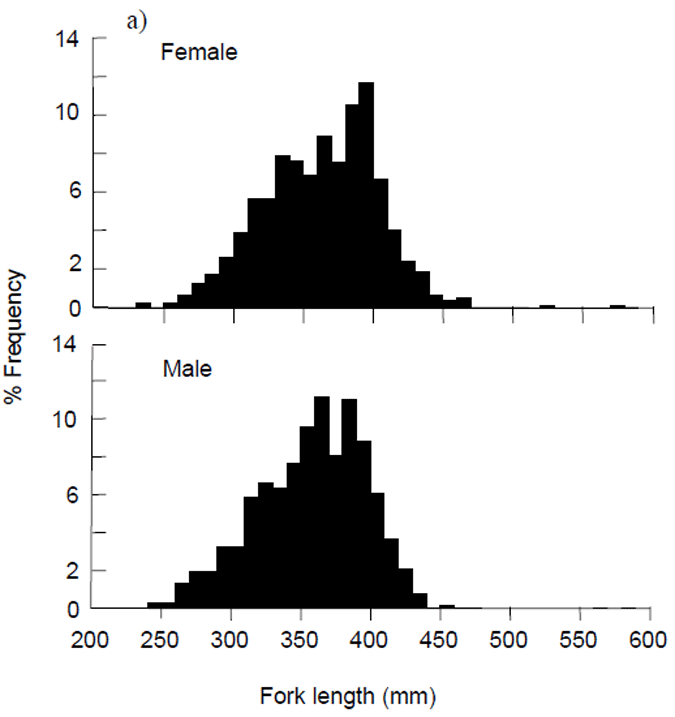
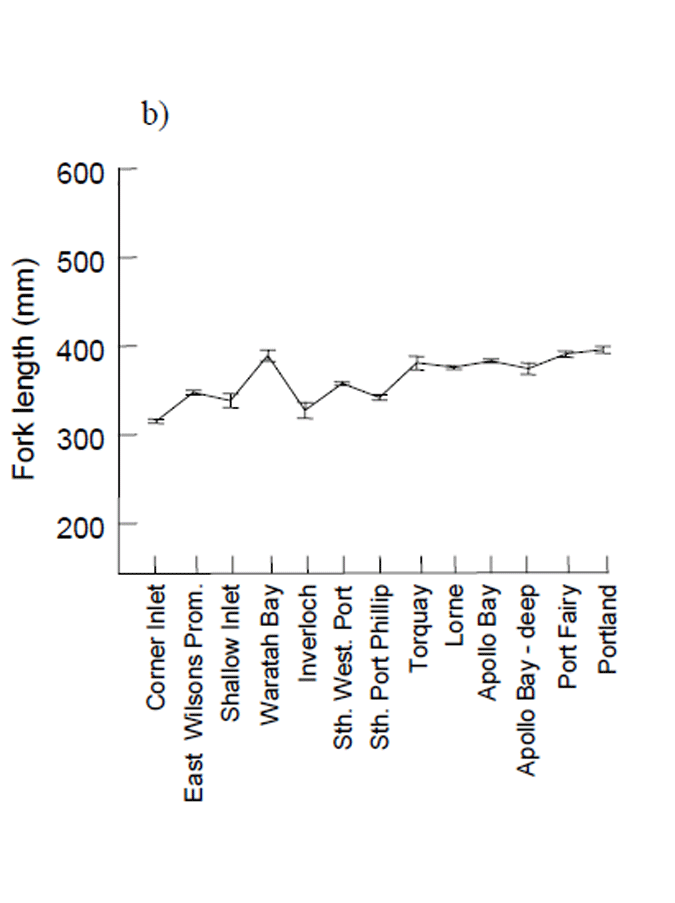
Figure 8. a) Length frequency distributions for female and male King George whiting sampled by recreational anglers from Victorian waters and examined for reproductive condition, November 2002 - June 2004, and b) Mean length (± 1 standard error) of King George whiting sampled by recreational anglers compared across sampling locations from east to west.
Age / length relationship
The age/length relationship of samples collected by recreational anglers during this project is plotted in Fig. 9a. Because the age/length data from this project was comprised mostly of fish over 35 cm FL and 3 year of age we plotted the von-Bertalanffy growth curve for data from all fish aged from Victorian waters by the Central Ageing Facility at PIRVic – Marine and Freshwater Systems (Fig. 9b). The data shows that by 4 years of age most fish in coastal waters are approaching 40 cm FL (Fig. 9ab). There-after the growth rate decreases and most 6 year old fish are below about 45 cm FL (Fig. 9ab). Beyond age 6 there is little age data for Victorian populations, however, the available data suggests that fish larger than 50 cm fork length are likely to be in vicinity of 10 years.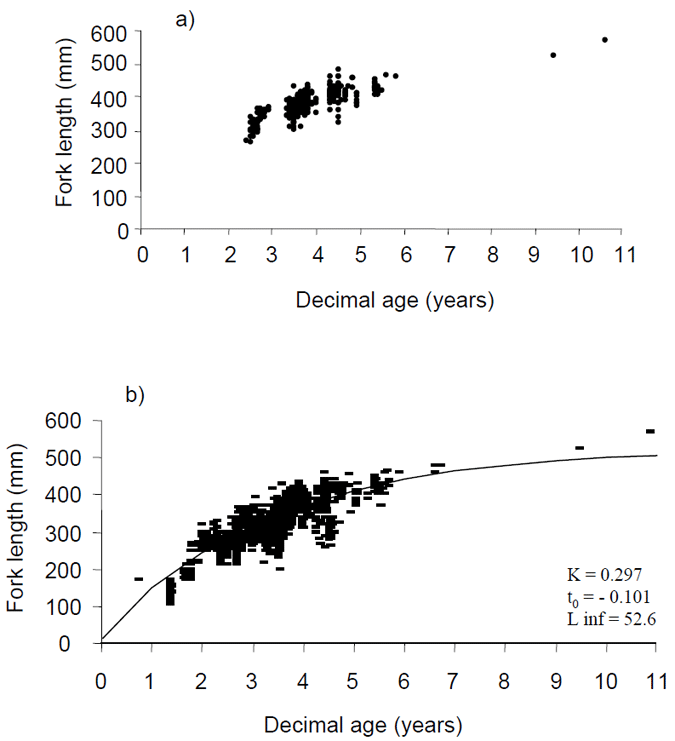
Figure 9. a) Age versus length of King George whiting sampled by recreational anglers in Victorian ocean waters and the entrance regions of Port Phillip and Western Port Bays, November 2002 - June 2003, b) age versus length with von-Bertalanffy growth curve fitted for all King George whiting aged from Victorian waters including bays and inlets.
Decimal age is the age-at-capture in decimal years based on a birth date of the 1st July.
Discussion
This investigation has provided limited evidence for the occurrence of spawning by King George whiting in Victorian waters. Of the approximately 1600 specimens examined we found complete gonad maturation in only one female and did not find any fully mature males. Although we found several females in a highly developed reproduction state there was no evidence that these fish had spawned prior to capture or were about to spawn. These results suggest that King George whiting are not spawning where recreational anglers target them along the Victorian coast, and importantly, they imply that spawning King George whiting are not exploited by Victorian anglers. Our observation supports the anecdotal information supplied by recreational anglers during the project that suggests King George whiting in roe are rarely captured.
Although spawning adults were largely absent from recreational catches, we cannot rule out the possibility that spawning may occur in areas not fished by recreational anglers. Previous investigations of the spawning behaviour of King George whiting in Western Australia have suggested that spawning may occur in depths from 6 to at least 50 m (Hyndes et al. 1998). Although research in South Australia suggests that spawning may occur predominantly in deeper waters, it has been observed in waters shallower than 10 m (Fowler et al. 2000). Although King George whiting were collected over a broad geographic range, the majority of samples obtained from recreational anglers came from water depths of less than 20m. This reflected the fishing behaviour of Victorian anglers, who target King George whiting in shallow water where they can visualise the bottom habitat.
Although King George whiting are known to occur in deeper water along the Victorian coast, little is known about their distribution or abundance in depths greater than 20 m. To extend our sampling into deeper water habitats we obtained samples from depths of 30 – 40 m off Apollo Bay and Wilsons Promontory. These samples were derived from commercial Danish seine fishermen during the expected spawning period (May/June). Similar to the inshore samples there was an absence of both fully developed males and females. Further sizes of fish from offshore were similar to inshore samples. Our sampling in deeper waters was, however, both spatially and temporally limited. Research in both Western and South Australia suggests that spawning grounds are very restricted in space (Hyndes et al. 1998, Fowler et al. 1999, 2000a). If spawning does occur in specific areas in deep water, finding these areas would require considerable effort beyond the scope of the current project.
The size range of fish sampled during this study encompassed that of previous studies where spawning of King George whiting has been observed (Hyndes et al. 1998, Fowler et al. 1999). Therefore, size should not have been a factor influencing the absence of spawning fish, however, the age composition of samples was interesting with regards to the lack of old fish and the narrow range of age classes present. In South Australia, King George whiting of all age classes from 2 to 17 years have been sampled simultaneously in coastal waters (Fowler and McGarvey 2000, Fowler et al. 2000a). It is possible that age may have been an important factor influencing the lack of spawning, although research in South Australia has demonstrated that King George whiting of all ages above 3 years could be reproductively active (Fowler et al. 1999).
Research in South Australia, however, showed that in some locations, although many fish of 3 to 5 years age showed highly developed gonads, they did not appear to spawn (Fowler and McGarvey 2000, Fowler et al. 1999). This is similar to what we observed in samples of developed fish we examined from Victorian waters, where there were yolked oocytes, and yolked oocytes in atresia, but no post-ovulatory follicles (Fig. 3a). This indicates development close to spawning, but that spawning did not occur. This phenomenon is not uncommon in other fish species (Farley and Davis 1998), and suggests an absence of the cues necessary to trigger spawning (Fowler et al. 1999). The discrete spawning areas that were identified in South Australia were characterised by a broad mix of size and age classes (Fowler and McGarvey 2000). Fowler and McGarvey (2000) suggested two possible cues that might be required for spawning to occur; the presence of older mature fish and habitat of an exposed nature characterised by deeper water and patches of reef. The absence of older mature fish may be one reason for the lack of spawning observed during this study. Fowler and McGarvey (2000) also found that ripe males were always present with spawning females. In contrast we did not find any ripe males in our collections. Further, unlike the South Australia study where well developed females (macroscopic stage 3) were found in most areas, irrespective of the size or age distribution (Fowler and McGarvey 2000), we found very few females of macroscopic stage 3 in any area.
Without a more detailed investigation of the King George whiting inhabiting deeper waters along the Victorian coast we cannot be conclusive as to the importance of King George whiting spawning in Victorian coastal waters. The only known locations for King George whiting spawning along the south-eastern coastline of Australia are situated in South Australian waters, in particular around Kangaroo Island (Fowler and McGarvey 2000). However, there has been no investigation of King George whiting populations in waters between Kangaroo Island and Victoria, and modelling of larval drift would suggest that King George whiting recruiting into Victorian bays and inlets could come from unknown spawning grounds in this region (Jenkins et al. 2000).
Our results do not contradict this prediction, and provide several lines of evidence that support this idea. In particular, the lack of old fish and the observation that the age and size of fish increased with distance to the west. Due to the low fishing pressure in Victorian coastal waters and the longevity of King George whiting (up to 17 years), the lack of older fish is unlikely to be a result of high natural or fishing mortality. The lack of older fish is consistent with a gradual migration from the nursery grounds of Port Phillip Bay and Western Port back to spawning grounds in South Australia.
Discussions with fishermen along the Victorian coastline also suggest that the coastal fishery is characterised by short-term temporal dynamics similar to that of the Port Phillip Bay fishery. This is consistent with fish passing through coastal areas and not remaining resident for long periods. One way of confirming the migration of King George whiting from Victorian waters to spawning areas in South Australia is tag/recapture. Currently few King George whiting are tagged in Victorian waters. However, the few fish that have been tagged in Port Phillip Bay and recaptured outside the Bay, have been recaptured in areas well to the west of Port Phillip Bay (Patrick Coutin, personnel communication). Demonstration of migration of adult King George whiting from Victoria to South Australia would be critical to understanding the importance of spawning in Victorian waters.
IMPLICATIONS FOR MANAGEMENT
Spawning King George whiting are not exploited by anglers in Victorian coastal waters. However, the question of how dependent the Victorian fishery is on the exploited spawning stocks in South Australian waters remains unclear. While it is possible that spawning does occur in Victorian waters at some locations, our results are more consistent with the idea that spawning is not extensive, and that fish migrate away from Victorian fishing areas with age. It would be very difficult to identify highly localised spawning areas, particularly in deeper waters, however, there are other means for clarifying the reason for the lack of spawning fish found during this study. The simplest approaches would involve tag/recapture and or otolith chemistry studies to determine in Victorian fish are migrating back to South Australia. This question of migration will need to be resolved before managers can be confident of the link between the Victorian fishery and the spawning stock in South Australia.
Conclusions
Although we found evidence of spawning, our results provide no indication of extensive spawning by King George whiting along the Victorian coastline. Our results in combination with previous work lead to the generation of two hypotheses to explain the lack of spawning King George whiting observed in Victorian coastal waters during this project. The first hypothesis is that King George whiting spawn in highly localised, deep-water habitats, and are therefore difficult to detect with angler-based surveys. The second hypothesis is that most King George whiting that spend their juvenile years in Victorian bays and inlets migrate back to the major spawning grounds in eastern South Australia. In order to determine which of the hypotheses is correct future research should involve more extensive sampling of deeper water habitats and/or investigation of adult migration behaviour to determine if Victorian juveniles are returning to South Australian waters as adults.
Acknowledgements
We would like to acknowledge the generous funding provided by the Recreational Fishing License Grants Program and Fisheries Victoria. We would also like to thank the many recreational anglers who donated frames, expressed interest and spread the word about the search for spawning King George whiting. We also greatly appreciate the assistance of Fisheries Officers who provided support for the collection of frames, and provision of storage facilities in regional areas. PIRVic staff who assisted in this work included; Andrew Kidd, David Hatton, Daniel Grixti, Ryan Bonner, Leanne Gunthorpe and David McKeown.
We would also like the to thank the Central Ageing Facility and Mr. Simon Conron at PIRVic – MaFS for supply the extra age data.
Recreational anglers involved in sample collection at Apollo Bay – May 2003
References
Cockrum K S, Jones G K (1992) The reproductive biology and fecundity of King George whiting (Sillaginodes punctata) in South Australian waters, 1953-1988. South Australian Research and Development Institute. No. 25.
Farely JH, Davis TLO (1998) Reproductive dynamics of southern bluefin tuna, Thunnus maccoyii, in the Indian ocean south of the Sunda Islands.Fishery Bulletin 96, 223-236
Fowler AJ, Short DA (1998) Validation of age determination from otoliths for King George whiting (Perciformes: Sillaginodes punctata). Marine Biology 130, 577-578
Fowler AJ, McLeay L, Short DA (1999) Reproductive mode and spawning information based on gonad analysis for the King George whiting (Percoidei: Sillaginidae) from South Australia. Marine and Freshwater Research 50, 1- 14.
Fowler AJ, McGarvey R (2000) 'Development of an integrated fisheries management model for King George whiting (Sillagnodes punctata) in South Australia'. FRDC project 95/008 Final Report.
Fowler AJ, McLeay L, Short DA (2000a) Spatial variation in size and age structures and reproductive characteristics of the King George whiting (Percoidei: Sillaginidae) in South Australian waters. Marine and Freshwater Research 51, 11-22.
Fowler AJ, Black KP, Jenkins GP (2000b) Determination of spawning areas and larval advection pathways for King George whiting in southeastern Australia using otolith microstructure and hydrodynamic modelling. II. South Australia. Marine Ecology Progress Series 199, 243-254
Hunter JR, Macewicz BJ (1985) Measurement of spawning frequency in multiple spawning fishes. In 'An egg production method for estimating spawning biomass of pelagic fish: application to the northern anchovy,Engraulis mordax'. (Ed. R. Lasker,). pp. 79-94, U.S. Department of Commerce.
Hyndes GA, Platell ME, Potter IC, Lenanton RCJ (1998) Age composition, growth, reproductive biology and recruitment of king George whiting (Sillaginodes punctata) in south-western Australia. Fishery Bulletin 96, 258-270.
Jenkins GP, May HMA (1994) Variation in settlement and larval duration of King George Whiting, Sillaginodes punctata (Sillaginidae), in Swan Bay, Victoria, Australia. Bulletin of Marine Science 54 (1), 281-296.
Jenkins GP, Black KP, Hamer P A (2000) Determination of spawning areas and larval advection pathways for King George whiting in southeastern Australia using otolith microstructure and hydrodynamic modelling. I. Victoria. Marine Ecology Progress Series 199, 231- 242.
Jones GK, Hall KM, Staniford A (1990) 'The South Australian marine scalefish fishery: stock assessment, economics, management'. South Australian Department of Fisheries Green Paper.
Kailola PJ, Williams MJ, Stewart PC, Reichelt RE, McNee A, Grieve C (1993) 'Australian Fisheries Resources'. Bureau of Resource Sciences, Department of Primary Industries and Energy, and Fisheries Research and Development Corporation, Canberra, Australia. Lunar LG (1968) 'Manual of histological staining methods of the armed forces institute of pathology'. McGraw-Hill, Sydney
McGlennon D, Kinloch MA (1997) 'Resource allocation in the South Australian marine scalefish fishery'. FRDC project 93/249, Final Report
Knuckey IA, Sivakumaran KP (2001) Reproductive characteristics and per-recruit analyses of blue warehou (Seriolella brama): implications for the South East Fishery of Australia. Marine and Freshwater Research 52, 578- 87.
Appendix 1 – Details of publicity and extension related to the project
| Publicity | Date |
|---|---|
| Press release – all major press outlets in Victoria | Sept 02 |
| Radio | |
| Radio interview - ABC Melbourne on Derrick Geel's program | Sept 02 |
| Radio interview - ABC Warnambool | Sept 02 |
| Radio interview - 3YB Coloc | Sept 02 |
| Radio interview – Pulse FM, Fish Victoria radio show | May 03 |
| Print media | |
| Newspaper article – Herald Sun | Sept 02 |
| Newspaper article – Echo , circulated Bellarine Peninsula and south-west coast to Apollo Bay | Sept 02 |
| Newspaper article - Portland Observer , circulated far western Victoria coastal region | Apr 03 |
| Newspaper article – Yarram Standard, circulated south east Gippsland, Wilsons Promontory | May - 03 |
| Newsletters | |
| Newsletter article – Rip Rumour, circulated Queenscliff- Point Lonsdale | Oct 02 |
| VRfish newsletter | Jan 03 |
| Fish Fax – Fish Fax article no. 85, circulated angling clubs, recreational fishing media | Feb 03 |
| Fisheries Note - publication no. 0533, circulated to VRFish, Seafood Industry Victoria (SIV), Victorian Marine | Feb 03 |
| Parks Authority (VNPA), Fish Care, Fisheries Officers, all PIRVic staff, angling representatives, DPI web page | |
| Fisheries Note – publication no. 0540, circulated as above | Feb 03 |
| Fisheries Note – publication no 0547,circulated as above | May 03 |
| Flyers | |
| Posted in petrol station and tackle shops in the following locations: Queenscliff, Point Lonsdale, Sorrento, Torquay, Lorne, Kennet River, Apollo Bay, Warnambool, Portland, Melbourne, Foster, Lakes Entrance, Marlo, Mallacoota | Oct 02 – Feb |
| Oral Presentations | |
| Geelong Angling Club | Sept 02 |
| Snapper Point Angling Club (Mornington) | Oct 02 |
| Lorne Aquatic Club | Nov 02 |
| Lakes Entrance Angling Club | Jan 03 |
| Torquay Angling Club | Feb 03 |
| Wonthaggii Angling Club | Mar 03 |
| Fish Care Training Workshop, Queenscliff | May 03 |
| Apollo Bay Fishermens Group | May 03 |
| Walkerville Angling Club | Feb 04 |
| Victorian Fishcare Conference | June 04 |
| Attendance and displays at fishing competitions | |
| Tea Tree Snapper competition, Mornington, run by Snapper Pt. Angling Club | Nov 02 |
| Portland ANSA competition | Jan 03 |
| Western Port whiting challenge | Feb 03 |
| Tea Tree Snapper competition | Nov 03 |
| Portland ANSA competition | Jan 03 |
| Western Port whiting challenge | Feb 04 |
| Walkerville Angling Club monthly competition | Mar 04 |
| Clubs involved in "King George whiting search for a spawner competition" | |
| Torquay Angling Club | Mar-June 03 |
| Walkerville Angling Club | Mar-June 03 |
| Warnambool Angling Club | Mar-June 03 |
| Portland Sport and Game Fishing Club | Mar-June 03 |
| Portland Angling Club | Mar-June 03 |
| Port Albert Light Game and Sportfishing Club | Mar-June 03 |
| Other | |
| Display at "The Great Outdoors and Fishing Expo" | Oct 03 |
Appendix 2 – Keys used for reproductive staging of King George whiting
a) macrosopic stage key (taken from Fowler and McGarvey 2000)
| Stage | Macroscopic description |
|---|---|
| 1 Immature | Female – Ovaries small, undeveloped, clear, jelly-like or glassy, grey pink Male – Testes very small, flat, black and thread like |
| 2 Developing | Female – Ovaries small, opaque, light yellow in colour; individual oocytes not discernible Male – Testes flat/rounder in shape, testes occupy 20 – 70% of the length of the body cavity |
| 3 Developed | Female – Ovaries relatively large and quite turgid, yellow-orange with individual oocytes discernible Male- Testes lobed in formation, marked groove in the middle of each testis visible, testes occupy 40 - 70% of the length of the body cavity, creamy or white colouration with milt sometimes present |
| 4 Gravid or/ running ripe | Female – Ovaries large, orange, clear hydrated oocytes visible among opaque oocytes, oocytes may be ovulated Male – Testes very large and lobed/multilobed, testes occupy 40 – 70% of the body cavity, free flowing milt, testes white or pink and sometimes bloodshot |
| 5 Regressing or resting | Female – Ovaries small to medium, mustard yellow/orange/reddish, more flaccid than previous stages, and with granular appearance Male – Testes very bloodshot, testes occupy 20 – 50% of the length of the body cavity, milt sometimes present, testes brownish and rubbery as they regress to resting stage |
b) microscopic stage key for female ovaries (taken from Knuckey and Sivakumaran 2001)
| Stage | Histological description |
|---|---|
| 1 - Chromatin nucleolar stage | Very small oocytes, nucleus surrounded by a thin layer of dark-blue-stained cytoplasm |
| 2 - Perinucleolar stage | Oocyte size increases slightly as dark-blue-stained cytoplasm thickens, nucleoli appear at the periphery of nucleus |
| 3 - Cortical alveoli stage | Appearance of cortical alveoli in pale-blue-stained cytoplasm, pink-stained zona radiata distinguishable, oil vesicles appearing, lampbrush chromosomes often visible in the nucleus |
| 4 - Yolk stage | Marked increase in oocyte size, cytoplasm filled with pink-stained yolk granules, cortical alveoli and oil vesicles increase in size and number |
| 5 - Nuclear migration stage | Migration of nucleus to periphery of oocyte, fusion of yolk granules into yolk plates; fusion of oil vesicles into the oil droplet |
| 6 - Hydration stage | Further increase in size of oocytes, all yolk granules fused into a few plates |
| Other features | Description |
| Postovulatory follicle (POF) (new) | Remaining follicle soon after ovulation. It is large, highly convoluted with an obvious lumen, and may contain fine granular material. The layered nature of both cell types (thecal and granulosa) remains intact in lumen |
| Postovulatory follicle (old) | Convoluted nature much less apparent, lumen much reduced, even closed, and the thecal and granulosa cells no longer retain their orderly arrangement |
| ∞−atretic oocyte | Zona radiata dissolves, oocyte shape loses integrity yolk globules begin to disintegrate and are less regular in shape |
